当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ontogeny of Hepatic Transporters and Drug-Metabolizing Enzymes in Humans and in Nonclinical Species
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2021-04-01 , DOI: 10.1124/pharmrev.120.000071 B D van Groen 1 , J Nicolaï 1 , A C Kuik 1 , S Van Cruchten 1 , E van Peer 1 , A Smits 1 , S Schmidt 1 , S N de Wildt 1 , K Allegaert 1 , L De Schaepdrijver 1 , P Annaert 2 , J Badée 1
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2021-04-01 , DOI: 10.1124/pharmrev.120.000071 B D van Groen 1 , J Nicolaï 1 , A C Kuik 1 , S Van Cruchten 1 , E van Peer 1 , A Smits 1 , S Schmidt 1 , S N de Wildt 1 , K Allegaert 1 , L De Schaepdrijver 1 , P Annaert 2 , J Badée 1
Affiliation
|
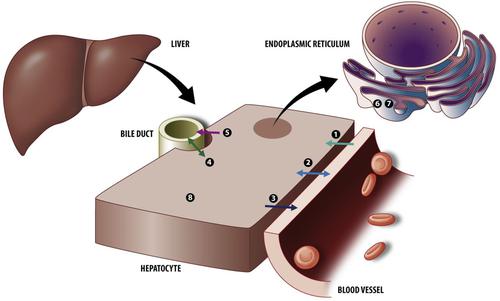
The liver represents a major eliminating and detoxifying organ, determining exposure to endogenous compounds, drugs, and other xenobiotics. Drug transporters (DTs) and drug-metabolizing enzymes (DMEs) are key determinants of disposition, efficacy, and toxicity of drugs. Changes in their mRNA and protein expression levels and associated functional activity between the perinatal period until adulthood impact drug disposition. However, high-resolution ontogeny profiles for hepatic DTs and DMEs in nonclinical species and humans are lacking. Meanwhile, increasing use of physiologically based pharmacokinetic (PBPK) models necessitates availability of underlying ontogeny profiles to reliably predict drug exposure in children. In addition, understanding of species similarities and differences in DT/DME ontogeny is crucial for selecting the most appropriate animal species when studying the impact of development on pharmacokinetics. Cross-species ontogeny mapping is also required for adequate translation of drug disposition data in developing nonclinical species to humans. This review presents a quantitative cross-species compilation of the ontogeny of DTs and DMEs relevant to hepatic drug disposition. A comprehensive literature search was conducted on PubMed Central: Tables and graphs (often after digitization) in original manuscripts were used to extract ontogeny data. Data from independent studies were standardized and normalized before being compiled in graphs and tables for further interpretation. New insights gained from these high-resolution ontogeny profiles will be indispensable to understand cross-species differences in maturation of hepatic DTs and DMEs. Integration of these ontogeny data into PBPK models will support improved predictions of pediatric hepatic drug disposition processes.
中文翻译:

人类和非临床物种中肝转运蛋白和药物代谢酶的个体发育
肝脏是一个主要的消除和解毒器官,决定着内源性化合物、药物和其他外源性物质的暴露。药物转运蛋白 (DTs) 和药物代谢酶 (DMEs) 是药物处置、功效和毒性的关键决定因素。围产期至成年期间其 mRNA 和蛋白质表达水平以及相关功能活动的变化会影响药物处置。然而,缺乏非临床物种和人类肝脏 DTs 和 DMEs 的高分辨率个体发育谱。同时,越来越多地使用基于生理学的药代动力学 (PBPK) 模型需要潜在个体发育特征的可用性,以可靠地预测儿童的药物暴露。此外,在研究发育对药代动力学的影响时,了解 DT/DME 个体发育的物种异同对于选择最合适的动物物种至关重要。在将非临床物种开发给人类的过程中,为了将药物处置数据充分翻译,还需要跨物种个体发育图谱。本综述提供了与肝脏药物处置相关的 DTs 和 DMEs 个体发育的跨物种定量汇编。在 PubMed Central 上进行了全面的文献检索:原始手稿中的表格和图表(通常在数字化之后)用于提取个体发育数据。来自独立研究的数据在被编入图表和表格以供进一步解释之前被标准化和标准化。从这些高分辨率个体发育谱中获得的新见解对于理解肝脏 DTs 和 DMEs 成熟的跨物种差异是必不可少的。将这些个体发育数据整合到 PBPK 模型中将支持改进对儿科肝脏药物处置过程的预测。
更新日期:2021-02-21
中文翻译:

人类和非临床物种中肝转运蛋白和药物代谢酶的个体发育
肝脏是一个主要的消除和解毒器官,决定着内源性化合物、药物和其他外源性物质的暴露。药物转运蛋白 (DTs) 和药物代谢酶 (DMEs) 是药物处置、功效和毒性的关键决定因素。围产期至成年期间其 mRNA 和蛋白质表达水平以及相关功能活动的变化会影响药物处置。然而,缺乏非临床物种和人类肝脏 DTs 和 DMEs 的高分辨率个体发育谱。同时,越来越多地使用基于生理学的药代动力学 (PBPK) 模型需要潜在个体发育特征的可用性,以可靠地预测儿童的药物暴露。此外,在研究发育对药代动力学的影响时,了解 DT/DME 个体发育的物种异同对于选择最合适的动物物种至关重要。在将非临床物种开发给人类的过程中,为了将药物处置数据充分翻译,还需要跨物种个体发育图谱。本综述提供了与肝脏药物处置相关的 DTs 和 DMEs 个体发育的跨物种定量汇编。在 PubMed Central 上进行了全面的文献检索:原始手稿中的表格和图表(通常在数字化之后)用于提取个体发育数据。来自独立研究的数据在被编入图表和表格以供进一步解释之前被标准化和标准化。从这些高分辨率个体发育谱中获得的新见解对于理解肝脏 DTs 和 DMEs 成熟的跨物种差异是必不可少的。将这些个体发育数据整合到 PBPK 模型中将支持改进对儿科肝脏药物处置过程的预测。









































 京公网安备 11010802027423号
京公网安备 11010802027423号