Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-02-21 , DOI: 10.1016/j.psep.2021.02.021 Zhenxin Jiang , Qingyan Chu , Haiyu Yang , RongRong Zhao , Yueman Yu , Ming Wang , Ransheng Liu
|
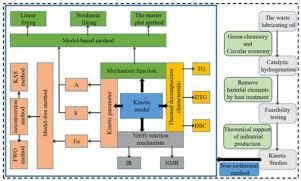
The present study focused on the establishment of a kinetic model for the removal of phosphorus and zinc from waste lubricating oil by pyrolysis. Zinc dialkyl dithiophosphate (ZDDP) was used as the model compound for the analysis. The pyrolysis of ZDDP was evaluated at different temperatures by infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. Moreover, thermogravimetry (TG), derivative thermogravimetry (DTG), and differential scanning calorimetry (DSC) curves were used to investigate the thermal decomposition characteristics of ZDDP at different heating rates. The apparent activation energies at different conversion rates were calculated by the Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) methods. Finally, the thermal decomposition mechanism at each stage was determined by a model-based approach. The results indicated that the thermal decomposition process of ZDDP could be described by a four-step thermal decomposition model. The average apparent activation energy calculated by the FWO method was 107.959 kJ mol−1, while that calculated by the KAS approach was 105.0681 kJ mol−1. Additionally, it was established that the decomposition induction stage (II), competitive reaction stage (III), and precipitation stage (IV) involved a power function model reaction (P4) mechanism, Avrami–Erofeev reaction (A3) mechanism, and Avrami–Erofeev reaction (A2) mechanism, respectively. Importantly, the predactyl factor A and reaction rate constant K were also obtained. The outcomes of this study provide a valuable basis for optimizing the process of industrial pyrolysis of waste lubricating oil.
中文翻译:

热解脱除废润滑油中磷和锌的动力学模型
本研究的重点是建立动力学模型,通过热解从废润滑油中去除磷和锌。二烷基二硫代磷酸锌(ZDDP)被用作分析的模型化合物。ZDDP的热解通过红外(IR)和核磁共振(NMR)光谱在不同温度下进行评估。此外,使用热重法(TG),微分热重法(DTG)和差示扫描量热法(DSC)曲线研究了ZDDP在不同加热速率下的热分解特性。表观活化能在不同转化率下是通过基辛格-赤平-Sunose(KAS)和弗林-沃尔-小泽(FWO)方法计算的。最后,通过基于模型的方法确定每个阶段的热分解机理。结果表明,ZDDP的热分解过程可以用四步热分解模型来描述。通过FWO方法计算的平均表观活化能为107.959 kJ mol-1,而通过KAS方法计算的是105.0681 kJ mol -1。此外,已确定分解诱导阶段(II),竞争反应阶段(III)和沉淀阶段(IV)涉及幂函数模型反应(P4)机制,Avrami–Erofeev反应(A3)机制和Avrami–分别发生Erofeev反应(A2)的机理。重要的是,还获得了前癸基因子A和反应速率常数K。这项研究的结果为优化废润滑油的工业热解工艺提供了有价值的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号