当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New insights into the facilitated dissolution and sulfidation of silver nanoparticles under simulated sunlight irradiation in aquatic environments by extracellular polymeric substances
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-2-3 , DOI: 10.1039/d0en01142h Yi Yang 1, 2, 3, 4, 5 , Shimei Zheng 6, 7, 8, 9 , Ruixuan Li 1, 2, 3, 4, 5 , Xin Chen 1, 2, 3, 4, 5 , Kunkun Wang 1, 2, 3, 4, 5 , Binbin Sun 1, 2, 3, 4, 5 , Yinqing Zhang 1, 2, 3, 4, 5 , Lingyan Zhu 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2021-2-3 , DOI: 10.1039/d0en01142h Yi Yang 1, 2, 3, 4, 5 , Shimei Zheng 6, 7, 8, 9 , Ruixuan Li 1, 2, 3, 4, 5 , Xin Chen 1, 2, 3, 4, 5 , Kunkun Wang 1, 2, 3, 4, 5 , Binbin Sun 1, 2, 3, 4, 5 , Yinqing Zhang 1, 2, 3, 4, 5 , Lingyan Zhu 1, 2, 3, 4, 5
Affiliation
|
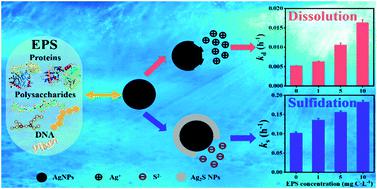
Due to their widespread application and consequent release into aquatic environments, silver nanoparticles (AgNPs) are widely present in aquatic environments. Extracellular polymeric substances (EPS) originating from aquatic organisms are naturally biogenic polymers and may interact with nanomaterials in the environment. In this study, the impacts of EPS harvested from Chlorella vulgaris on the oxidative dissolution and sulfidation of AgNPs were explored through a combination of spectral analyses and kinetic estimations. At various concentrations, algal EPS facilitated both the dissolution and sulfidation of AgNPs. The abundant aromatic groups and nitrogen containing groups were conducive to the adsorption of the algal EPS onto the surface of AgNPs. In addition, several kinds of reactive oxygen species (ROS) were generated from the EPS under light irradiation, and might actively react with AgNPs. Moreover, the abundant surface functional groups of EPS might complex with Ag+ ions. These could synergistically facilitate the dissolution of AgNPs. Besides, the adsorption of EPS on AgNPs reduced the electrostatic repulsion and favored the access of sulfide to react with AgNPs, and therefore promoted the sulfidation of AgNPs to a great extent. This study provides important insights into the interaction mechanisms between EPS and AgNPs and the results are helpful to predict the fate and risk of AgNPs in complicated natural aquatic environments.
中文翻译:

在水环境中模拟阳光照射下,细胞外聚合物质促进银纳米颗粒的溶解和硫化的新见解
由于其广泛应用并随后释放到水生环境中,银纳米颗粒(AgNPs)广泛存在于水生环境中。源自水生生物的细胞外聚合物(EPS)是天然的生物聚合物,可能与环境中的纳米材料相互作用。在这项研究中,从小球藻收获的EPS的影响通过光谱分析和动力学估算相结合的方法研究了AgNPs的氧化溶解和硫化反应。在不同浓度下,藻类EPS促进了AgNP的溶解和硫化。丰富的芳族基团和含氮基团有助于藻类EPS吸附到AgNPs的表面。此外,在光照射下,EPS产生了几种活性氧(ROS),并可能与AgNPs主动反应。此外,EPS的丰富表面官能团可能会与Ag +络合离子。这些可以协同促进AgNP的溶解。此外,EPS在AgNPs上的吸附降低了静电排斥力,有利于硫化物与AgNPs反应,从而在很大程度上促进了AgNPs的硫化。这项研究提供了重要的见解EPS和AgNPs之间的相互作用机制,结果有助于预测复杂自然水生环境中AgNPs的命运和风险。
更新日期:2021-02-19
中文翻译:

在水环境中模拟阳光照射下,细胞外聚合物质促进银纳米颗粒的溶解和硫化的新见解
由于其广泛应用并随后释放到水生环境中,银纳米颗粒(AgNPs)广泛存在于水生环境中。源自水生生物的细胞外聚合物(EPS)是天然的生物聚合物,可能与环境中的纳米材料相互作用。在这项研究中,从小球藻收获的EPS的影响通过光谱分析和动力学估算相结合的方法研究了AgNPs的氧化溶解和硫化反应。在不同浓度下,藻类EPS促进了AgNP的溶解和硫化。丰富的芳族基团和含氮基团有助于藻类EPS吸附到AgNPs的表面。此外,在光照射下,EPS产生了几种活性氧(ROS),并可能与AgNPs主动反应。此外,EPS的丰富表面官能团可能会与Ag +络合离子。这些可以协同促进AgNP的溶解。此外,EPS在AgNPs上的吸附降低了静电排斥力,有利于硫化物与AgNPs反应,从而在很大程度上促进了AgNPs的硫化。这项研究提供了重要的见解EPS和AgNPs之间的相互作用机制,结果有助于预测复杂自然水生环境中AgNPs的命运和风险。











































 京公网安备 11010802027423号
京公网安备 11010802027423号