当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Structure of Bimetallic NiW/SiO2 Catalysts and Its Consequences on Selective Deoxygenation of m-Cresol to Toluene
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-18 , DOI: 10.1021/acscatal.0c05560 Feifei Yang 1, 2 , Mallikharjuna Rao Komarneni 2 , Nicole J. Libretto 3 , Liwen Li 1 , Wei Zhou 1 , Jeffrey T. Miller 3 , Qingfeng Ge 4 , Xinli Zhu 1 , Daniel E. Resasco 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-18 , DOI: 10.1021/acscatal.0c05560 Feifei Yang 1, 2 , Mallikharjuna Rao Komarneni 2 , Nicole J. Libretto 3 , Liwen Li 1 , Wei Zhou 1 , Jeffrey T. Miller 3 , Qingfeng Ge 4 , Xinli Zhu 1 , Daniel E. Resasco 2
Affiliation
|
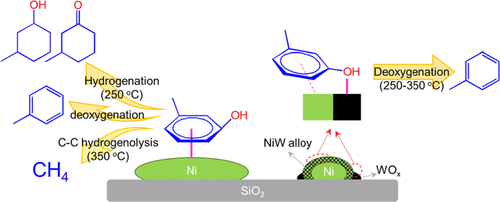
As a non-noble metal, Ni could offer significant economic advantages if used as a catalyst for hydrodeoxygenation (HDO) of lignin-derived phenolics to produce aromatics. However, on unmodified Ni catalysts, the desirable direct deoxygenation reaction must compete with high rates of phenyl-ring hydrogenation and C–C hydrogenolysis reactions, which lead to low aromatics yields. Here, we report on a bimetallic NiW/SiO2 (W/Ni = 1) prepared by coimpregnation that shows an HDO reaction rate of m-cresol almost an order of magnitude higher than that on Ni/SiO2 at 350 °C and 1 atm H2. More importantly, under these conditions, this catalyst exhibits a complete inhibition of CH4 formation, while at a temperature as low as 250 °C, the dominant product is still toluene, with minimal formation of ring-saturation products. To elucidate the structure of this catalyst, a detailed characterization was performed by combination of several techniques. It was found that the calcined NiW/SiO2 exhibits a large extent of Ni–W oxide interaction. After reduction at 500 °C, a thin NiW alloy shell with a small Ni core and WOx in close proximity are formed, with a strong interaction between Ni and adjacent W species. The electronic modifications of Ni and W species were monitored by X-ray photoelectron spectroscopy and it was found that these interactions alter the surface properties of the alloy, resulting in significantly weakened CO chemisorption. This unique structure provides a balanced hydrogenation, oxophilicity, and C–O cleavage activity, which result in a significantly improved rate and selectivity toward toluene with inhibition of CH4 and hydrogenation product formation.
中文翻译:

阐明双金属的NiW /二氧化硅的结构2的催化剂及其在选择性脱氧后果米到甲苯甲酚
作为一种非贵金属,如果将Ni用作木质素衍生的酚的加氢脱氧(HDO)催化剂以生产芳族化合物,则Ni可以提供显着的经济优势。但是,在未改性的Ni催化剂上,理想的直接脱氧反应必须与苯环加氢和C–C氢解反应的高速率相竞争,这导致芳烃收率低。在这里,我们的双金属的NiW /报告的SiO 2通过共浸渍制备(W / NI = 1),该节目的HDO反应速率米甲酚几乎一个数量级比对Ni /二氧化硅更高2在350℃和1个atm H 2。更重要的是,在这些条件下,该催化剂表现出对CH 4的完全抑制虽然在低至250°C的温度下仍能形成,但主要产物仍是甲苯,而环饱和产物的形成极少。为了阐明该催化剂的结构,通过几种技术的结合进行了详细的表征。结果发现,煅烧后的NiW / SiO 2表现出很大程度的Ni-W氧化物相互作用。在500°C下还原后,会形成一个薄的NiW合金外壳,带有小的Ni核和WO x在Ni和相邻的W物种之间有很强的相互作用,形成了紧密相邻的区域。通过X射线光电子能谱监测Ni和W物种的电子修饰,发现这些相互作用改变了合金的表面性能,导致CO化学吸附显着减弱。这种独特的结构提供了平衡的加氢,亲氧性和C–O裂解活性,从而显着提高了甲苯的合成速率和选择性,同时抑制了CH 4和加氢产物的形成。
更新日期:2021-03-05
中文翻译:

阐明双金属的NiW /二氧化硅的结构2的催化剂及其在选择性脱氧后果米到甲苯甲酚
作为一种非贵金属,如果将Ni用作木质素衍生的酚的加氢脱氧(HDO)催化剂以生产芳族化合物,则Ni可以提供显着的经济优势。但是,在未改性的Ni催化剂上,理想的直接脱氧反应必须与苯环加氢和C–C氢解反应的高速率相竞争,这导致芳烃收率低。在这里,我们的双金属的NiW /报告的SiO 2通过共浸渍制备(W / NI = 1),该节目的HDO反应速率米甲酚几乎一个数量级比对Ni /二氧化硅更高2在350℃和1个atm H 2。更重要的是,在这些条件下,该催化剂表现出对CH 4的完全抑制虽然在低至250°C的温度下仍能形成,但主要产物仍是甲苯,而环饱和产物的形成极少。为了阐明该催化剂的结构,通过几种技术的结合进行了详细的表征。结果发现,煅烧后的NiW / SiO 2表现出很大程度的Ni-W氧化物相互作用。在500°C下还原后,会形成一个薄的NiW合金外壳,带有小的Ni核和WO x在Ni和相邻的W物种之间有很强的相互作用,形成了紧密相邻的区域。通过X射线光电子能谱监测Ni和W物种的电子修饰,发现这些相互作用改变了合金的表面性能,导致CO化学吸附显着减弱。这种独特的结构提供了平衡的加氢,亲氧性和C–O裂解活性,从而显着提高了甲苯的合成速率和选择性,同时抑制了CH 4和加氢产物的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号