当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbene-Catalyzed Alkylation of Carboxylic Esters via Direct Photoexcitation of Acyl Azolium Intermediates
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-18 , DOI: 10.1021/acscatal.1c00165 Shi-Chao Ren 1 , Wen-Xin Lv 1 , Xing Yang 1 , Jia-Lei Yan 1 , Jun Xu 1, 2 , Fang-Xin Wang 1 , Lin Hao 1 , Huifang Chai 2 , Zhichao Jin 3 , Yonggui Robin Chi 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-18 , DOI: 10.1021/acscatal.1c00165 Shi-Chao Ren 1 , Wen-Xin Lv 1 , Xing Yang 1 , Jia-Lei Yan 1 , Jun Xu 1, 2 , Fang-Xin Wang 1 , Lin Hao 1 , Huifang Chai 2 , Zhichao Jin 3 , Yonggui Robin Chi 1, 3
Affiliation
|
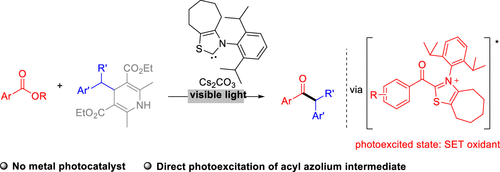
A carbene-catalyzed reductive coupling reaction of carboxylic esters and substituted Hantzsch esters is disclosed. Key steps of this reaction include one-electron reduction of a carbene catalyst-bound acyl azolium intermediate to generate the corresponding radical intermediate for subsequent alkylation reactions. The reaction is promoted by irradiation with visible light without the involvement of transition-metal photocatalysts. Mechanistic studies suggest that direct photoexcitation of the in situ formed acyl azolium intermediate is likely responsible for this light-induced one-electron-reduction process. Photoexcitation converts the acyl azolium intermediate to a single-electron oxidant, enabling single-electron oxidation of Hantzsch esters to generate radical intermediates. Our reactions work well for a broad range of aryl carboxylic ester and Hantzsch ester substrates. Sophisticated structures, including those present in medicines, can be incorporated into ketone molecules using our approach via very mild conditions that tolerate various functional groups.
中文翻译:

羰基偶氮中间体的直接光激发引起碳催化的羧酸酯烷基化
公开了羧酸酯和取代的汉茨tz酯的卡宾催化的还原偶联反应。该反应的关键步骤包括单电子还原卡宾催化剂所结合的酰基偶氮中间体,以产生相应的自由基中间体用于随后的烷基化反应。在不涉及过渡金属光催化剂的情况下,通过可见光照射可促进反应。机理研究表明,原位形成的酰基偶氮鎓中间体的直接光激发可能是这种光诱导的单电子还原过程的原因。光激发将酰基偶氮中间体转化为单电子氧化剂,从而使Hantzsch酯进行单电子氧化以生成自由基中间体。我们的反应适用于各种芳基羧酸酯和Hantzsch酯底物。可以使用我们的方法,通过非常温和的条件(可以耐受各种官能团)将复杂的结构(包括药物中存在的结构)并入酮分子中。
更新日期:2021-03-05
中文翻译:

羰基偶氮中间体的直接光激发引起碳催化的羧酸酯烷基化
公开了羧酸酯和取代的汉茨tz酯的卡宾催化的还原偶联反应。该反应的关键步骤包括单电子还原卡宾催化剂所结合的酰基偶氮中间体,以产生相应的自由基中间体用于随后的烷基化反应。在不涉及过渡金属光催化剂的情况下,通过可见光照射可促进反应。机理研究表明,原位形成的酰基偶氮鎓中间体的直接光激发可能是这种光诱导的单电子还原过程的原因。光激发将酰基偶氮中间体转化为单电子氧化剂,从而使Hantzsch酯进行单电子氧化以生成自由基中间体。我们的反应适用于各种芳基羧酸酯和Hantzsch酯底物。可以使用我们的方法,通过非常温和的条件(可以耐受各种官能团)将复杂的结构(包括药物中存在的结构)并入酮分子中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号