Journal of Membrane Science ( IF 8.4 ) Pub Date : 2021-02-19 , DOI: 10.1016/j.memsci.2021.119178 Yan Wang , Xiaoqin Qiao , Min Liu , Lei Liu , Nanwen Li
|
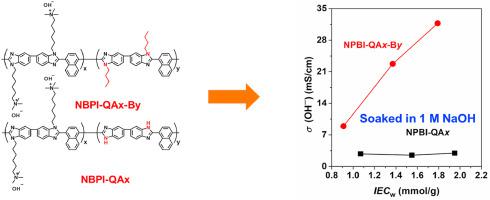
To investigate the effect of –NH− in polybenzimidazole (PBI) on the properties of PBI anion exchange membranes (AEMs), naphthalene based polybenzimidazole (NPBI) was partially grafted with cationic side chains to afford NPBI-QAx membrane, and then reacted with bromobutane to achieve fully grafting polymer namely NPBI-QAx-By. Surprisingly, after attaching n-butyl on the benzimidazole groups in NPBI backbones completely, the fully grafting polymer NPBI-QA55-B45 showed much higher hydroxide conductivity, achieving 31.7 mS/cm at 20 °C. This value was tenfold compared with that of NPBI-QA55 (2.96 mS/cm at 20 °C) membrane having similar IEC value without N-substitute. It was assumed that ionic interaction occurred between positively charged fixed cations and negatively charged benzimidazolide rings when the NPBI-QAx membranes were immersed in 1 M NaOH. FT-IR and XPS analysis on NPBI-QAx membranes confirmed that ionic interaction between positively charged fixed cations and negatively charged benzimidazolide rings occurred when treated in 1 M NaOH, while no obvious ionic interaction was observed when immersion in 1 M NaHCO3. However, the ionic interaction resulted in excellent stability of the membrane (NPBI-QA55) with –NH− groups, e.g. no obvious chemical structure degradation after alkaline stability test at 80 °C in 5 M NaOH for 760 h, while the NPBI-QA55-B45 membrane suffered from chemical degradations via ring opening reaction of NPBI backbones and nucleophilic substitution of pendant QA cations as confirmed by 1H NMR. Moreover, improved conductivity resulted in excellent fuel cell performance for NPBI-QA55-B45 membrane and the peak power density was about 260.1 mW/cm2. However, no fuel cell performance was obtained for the NPBI-QA55 membrane due to the formation of ionic interaction.
中文翻译:

–NH−对碱性燃料电池季铵化聚苯并咪唑阴离子交换膜的影响
为了研究聚苯并咪唑(PBI)中-NH-对PBI阴离子交换膜(AEMs)性能的影响,将基于萘的聚苯并咪唑(NPBI)部分接枝阳离子侧链,得到NPBI-QA x膜,然后与溴丁烷以实现完全接枝的聚合物,即NPBI-QA x -B y。令人惊讶的是,附加nNPBI主链中的苯并咪唑基团上的完全丁基-丁基,完全接枝的聚合物NPBI-QA55-B45显示出更高的氢氧化物电导率,在20°C下达到31.7 mS / cm。该值是具有类似IEC值但无N替代的NPBI-QA55(在20°C下为2.96 mS / cm)膜的十倍。假设将NPBI-QA x膜浸入1 M NaOH中时,带正电荷的固定阳离子与带负电荷的苯并咪唑环之间发生离子相互作用。在NPBI-QA x膜上进行的FT-IR和XPS分析证实,在1 M NaOH中处理时,带正电荷的固定阳离子与带负电荷的苯并咪唑啉环之间发生离子相互作用,而当浸入1 M NaHCO 3中时则未观察到明显的离子相互作用。。但是,离子相互作用导致带有-NH-基团的膜(NPBI-QA55)具有出色的稳定性,例如在80°C在5 M NaOH中进行760 h碱性稳定性测试后,没有明显的化学结构降解,而NPBI-QA55 -B45膜由于NPBI主链的开环反应和QA侧链阳离子的亲核取代而遭受化学降解,如1 H NMR所证实。此外,提高的电导率导致NPBI-QA55-B45膜具有出色的燃料电池性能,峰值功率密度约为260.1 mW / cm 2。但是,由于离子相互作用的形成,没有获得NPBI-QA55膜的燃料电池性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号