当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alpha helical surfactant-like peptides self-assemble into pH-dependent nanostructures
Soft Matter ( IF 2.9 ) Pub Date : 2021-2-10 , DOI: 10.1039/d0sm02095h Valeria Castelletto 1 , Jani Seitsonen , Janne Ruokolainen , Ian W Hamley
Soft Matter ( IF 2.9 ) Pub Date : 2021-2-10 , DOI: 10.1039/d0sm02095h Valeria Castelletto 1 , Jani Seitsonen , Janne Ruokolainen , Ian W Hamley
Affiliation
|
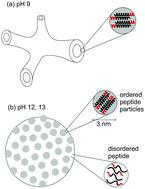
A designed surfactant-like peptide is shown, using a combination of cryogenic-transmission electron microscopy and small-angle X-ray scattering, to have remarkable pH-dependent self-assembly properties. Peptide Arg3-Leu12 (R3L12) forms a network of peptide nanotubes at pH 9 and below. These are associated with α-helical conformation in a “cross-α” nanotube structure, in which peptide dimers lie perpendicular to the nanotube axis, with arginine coated inner and outer nanotube walls. In contrast, this peptide forms decorated vesicular aggregates at higher pH values, close to the pKa of the arginine residues. These structures are associated with a loss of α-helical order as detected through X-ray scattering, circular dichroism and FTIR spectroscopy, the latter technique also revealing a loss of ordering of leucine side chains. This suggests a proposed model for the decorated or patchy vesicular structures that comprises disordered peptide as the matrix of the membrane, with small domains of ordered peptide dimers forming the minority domains. We ascribe this to a lipid-raft like phase separation process, due to conformational disordering of the leucine hydrophobic chains. The observation of the self-assembly of a simple surfactant-like peptide into these types of nanostructure is remarkable, and peptide R3L12 shows unique pH-dependent morphological and conformational behaviour, with the potential for a range of future applications.
中文翻译:

α螺旋表面活性剂样肽自组装成pH依赖的纳米结构
显示了一种设计的表面活性剂样肽,结合了低温透射电子显微镜和小角度X射线散射,具有显着的pH依赖性自组装特性。肽Arg 3 -Leu 12(R 3 L 12)在pH 9或以下形成肽纳米管网络。这些与“交叉-α”纳米管结构中的α-螺旋构象有关,其中肽二聚体垂直于纳米管轴,并带有精氨酸涂覆的内,外纳米管壁。相反,该肽在较高的pH值(接近p K a)时形成修饰的水泡聚集体。精氨酸残基。这些结构与通过X射线散射,圆二色性和FTIR光谱检测到的α螺旋顺序的损失有关,后者的技术也揭示了亮氨酸侧链的顺序的损失。这表明提出了一种修饰的或修补的囊泡结构的模型,该模型包含无序的肽作为膜的基质,有序的肽二聚体的小结构域形成少数结构域。由于亮氨酸疏水链的构象紊乱,我们将其归因于类脂筏相分离过程。观察到简单的表面活性剂样肽自组装为这些类型的纳米结构的现象非常明显,并且肽R 3 L 12 具有独特的pH依赖性形态和构象行为,具有潜在的未来应用范围。
更新日期:2021-02-18
中文翻译:

α螺旋表面活性剂样肽自组装成pH依赖的纳米结构
显示了一种设计的表面活性剂样肽,结合了低温透射电子显微镜和小角度X射线散射,具有显着的pH依赖性自组装特性。肽Arg 3 -Leu 12(R 3 L 12)在pH 9或以下形成肽纳米管网络。这些与“交叉-α”纳米管结构中的α-螺旋构象有关,其中肽二聚体垂直于纳米管轴,并带有精氨酸涂覆的内,外纳米管壁。相反,该肽在较高的pH值(接近p K a)时形成修饰的水泡聚集体。精氨酸残基。这些结构与通过X射线散射,圆二色性和FTIR光谱检测到的α螺旋顺序的损失有关,后者的技术也揭示了亮氨酸侧链的顺序的损失。这表明提出了一种修饰的或修补的囊泡结构的模型,该模型包含无序的肽作为膜的基质,有序的肽二聚体的小结构域形成少数结构域。由于亮氨酸疏水链的构象紊乱,我们将其归因于类脂筏相分离过程。观察到简单的表面活性剂样肽自组装为这些类型的纳米结构的现象非常明显,并且肽R 3 L 12 具有独特的pH依赖性形态和构象行为,具有潜在的未来应用范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号