Forensic Chemistry ( IF 2.6 ) Pub Date : 2021-02-17 , DOI: 10.1016/j.forc.2021.100314 Younis Abiedalla , Ahmad J. Almalki , Jack DeRuiter , C. Randall Clark
|
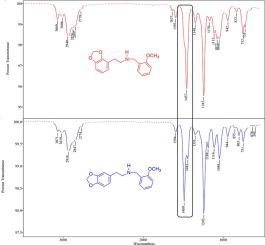
Twelve secondary amines representing structural analogues of the classic NBOMe category of novel psychoactive substances were prepared and evaluated in GC-EI-MS and vapor phase GC–IR studies. These compounds all contain the methylenedioxy group fused with the aromatic ring of the phenethyl moiety in the classic NBOMe chemical framework. One subseries of analogues, the three regioisomeric phenylisopropylamines, can be viewed as the N-methoxybenzyl analogues of 3,4-methylenedioxyamphetamine (MDA). Another subseries of three regioisomers contains the bromine and two methoxy groups at positions 2, 4 and 5 of the benzyl side of the molecule. The GC properties and the correlated EI-MS fragmentation products of these amines and some trifluoroacetamide derivatives were evaluated and compared to the traditional drug of abuse 25B-NBOMe. The compounds in this study are not currently known drugs of abuse and this proactive investigation provides data for differentiation of these analogues from the current NBOMe drugs of abuse. The observed GC elution order for the regioisomeric methoxybenzyl analogues on an Rxi®-17Sil MS phase was the 2-methoxy isomer eluting before the 3-substituted isomer, and the 4-substituted isomer eluting last. A similar comparison for the position of methylenedioxy fusion shows the 2,3-substituted isomer eluted before the 3,4-substituted isomer. All these secondary amines undergo EI fragmentation yielding the iminium cation via C–C bond cleavage of the phenethyl chain and the two benzylic cationic species. The mass spectra for the trifluoroacetyl derivatives in the bromodimethoxybenzyl substituted subseries provided additional structural information based on unique fragmentation processes. These characteristic ions are formed following initial hydrogen rearrangement within the molecular radical cation. The vapor phase infrared (vpIR) spectra confirm the position of substitution of the methylenedioxy, methoxy and bromodimethoxy groups.
中文翻译:

精神活性25X-NBOMe药物的亚甲二氧基苯基烷基胺类似物的GC-MS和GC-IR分析
制备了十二种代表新型精神活性物质经典NBOMe类别的结构类似物的仲胺,并在GC-EI-MS和气相GC-IR研究中进行了评估。这些化合物均含有在经典的NBOMe化学框架中与苯乙基部分的芳环稠合的亚甲基二氧基。一类类似物,即三种区域异构的苯基异丙基胺,可以看作是N3,4-亚甲基二氧基苯丙胺(MDA)的-甲氧基苄基类似物。三种区域异构体的另一个亚系列在分子的苄基侧的2、4和5位含有溴和两个甲氧基。评估了这些胺和一些三氟乙酰胺衍生物的GC特性以及相关的EI-MS裂解产物,并将其与传统的滥用药物25B-NBOMe进行了比较。这项研究中的化合物目前不是已知的滥用药物,这项积极的研究提供了将这些类似物与目前的NBOMe滥用药物区分开的数据。在Rxi?-17Sil MS相上观察到的区域异构体甲氧基苄基类似物的GC洗脱顺序是:2-甲氧基异构体先于3-取代异构体洗脱,而4-取代异构体最后洗脱。亚甲二氧基融合位置的类似比较显示,在3,4-取代异构体之前洗脱了2,3-取代的异构体。所有这些仲胺都经过EI断裂,通过苯乙基链和两个苄基阳离子物种的C–C键断裂产生亚胺阳离子。溴二甲氧基苄基取代的亚系列中三氟乙酰基衍生物的质谱图基于独特的断裂过程提供了其他结构信息。这些特征离子是在分子自由基阳离子内最初的氢重排之后形成的。气相红外(vpIR)光谱确定了亚甲二氧基,甲氧基和溴二甲氧基的取代位置。所有这些仲胺都经过EI断裂,通过苯乙基链和两个苄基阳离子物种的C–C键断裂产生亚胺阳离子。溴二甲氧基苄基取代的亚系列中三氟乙酰基衍生物的质谱图基于独特的断裂过程提供了其他结构信息。这些特征离子是在分子自由基阳离子内最初的氢重排之后形成的。气相红外(vpIR)光谱确定了亚甲二氧基,甲氧基和溴二甲氧基的取代位置。所有这些仲胺都经过EI断裂,通过苯乙基链和两个苄基阳离子物种的C–C键断裂产生亚胺阳离子。溴二甲氧基苄基取代的亚系列中三氟乙酰基衍生物的质谱图基于独特的断裂过程提供了其他结构信息。这些特征离子是在分子自由基阳离子内最初的氢重排之后形成的。气相红外(vpIR)光谱确定了亚甲二氧基,甲氧基和溴二甲氧基的取代位置。溴二甲氧基苄基取代的亚系列中三氟乙酰基衍生物的质谱图基于独特的断裂过程提供了其他结构信息。这些特征离子是在分子自由基阳离子内最初的氢重排之后形成的。气相红外(vpIR)光谱确定了亚甲二氧基,甲氧基和溴二甲氧基的取代位置。溴二甲氧基苄基取代的亚系列中三氟乙酰基衍生物的质谱图基于独特的断裂过程提供了其他结构信息。这些特征离子是在分子自由基阳离子内最初的氢重排之后形成的。气相红外(vpIR)光谱确定了亚甲二氧基,甲氧基和溴二甲氧基的取代位置。











































 京公网安备 11010802027423号
京公网安备 11010802027423号