Applied Surface Science ( IF 6.3 ) Pub Date : 2021-02-17 , DOI: 10.1016/j.apsusc.2021.149283 Yangge Guo , Guofeng Wang , Shuiyun Shen , Guanghua Wei , Guofeng Xia , Junliang Zhang
|
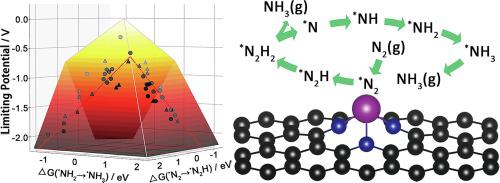
In this study, we performed the first-principles density functional theory calculations to systematically predict the activity of fifty-eight types of different single atom electrocatalysts on nitrogen doped graphene for electrochemical ammonia synthesis. Two strong linear relations were revealed among the reaction energies on these single atom structures, including positive correlation between the adsorption energy of N2H and the free energy change for *N2 transition to *N2H, as well as negative correlation between the adsorption energy of NH2 and the free energy change for *NH2 transition to *NH3. Using the developed scaling relations and some additional factors including nitrogen adsorption, hydrogen evolution reaction, water adsorption, ammonia desorption and structure stability, we have computationally identified six candidate structures as promising active sites for ammonia synthesis. Especially, V-N4/graphene was predicted to exhibit the best stability, the highest activity with the limiting potential of −0.71 V, and suppression of hydrogen evolution reaction.
中文翻译:

电化学合成氨用单原子催化剂的比例关系
在这项研究中,我们进行了第一原理密度泛函理论计算,以系统地预测58种类型的不同单原子电催化剂在氮掺杂石墨烯上的活性,以进行电化学氨合成。在这些单原子结构上的反应能量之间揭示了两个强线性关系,包括N 2 H的吸附能与* N 2跃迁至* N 2 H的自由能变化之间的正相关,以及在N 2 H跃迁至* N 2 H时的自由能变化之间的正相关。 NH 2的吸附能和* NH 2转变为* NH 3的自由能变化。利用已开发的比例关系以及包括氮吸附,氢释放反应,水吸附,氨解吸和结构稳定性在内的一些其他因素,我们通过计算确定了六个候选结构作为氨合成的有希望的活性位点。特别地,预测VN 4 /石墨烯表现出最佳的稳定性,在-0.71 V的极限电势下具有最高的活性,并且抑制了析氢反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号