当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation mechanisms of simple aliphatic amines under ozonation: a DFT study
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2021-1-24 , DOI: 10.1039/d0em00476f Qunfang Shen 1, 2, 3, 4, 5 , Yong Dong Liu 1, 2, 3, 4, 5 , Rugang Zhong 1, 2, 3, 4, 5
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2021-1-24 , DOI: 10.1039/d0em00476f Qunfang Shen 1, 2, 3, 4, 5 , Yong Dong Liu 1, 2, 3, 4, 5 , Rugang Zhong 1, 2, 3, 4, 5
Affiliation
|
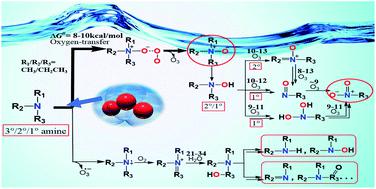
Aliphatic amines as common constituents of dissolved organic nitrogen (DON) exhibit high reactivity during ozonation; however, our understanding of their degradation mechanisms is very limited. In this study, methylamine (MA) and ethylamine (EA), as well as their secondary and tertiary amines (DMA, DEA, TMA and TEA) were chosen as aliphatic amine models and their degradation mechanisms during ozonation were investigated by using the DFT method. The oxygen-transfer reaction occurs initially and rapidly in the ozonation of all the above amines with a ΔG≠ value of 8–10 kcal mol−1 in great agreement with the experimental rate constant of 104 to 107 M−1 s−1. Moreover, N-oxide as the main degradation product for tertiary amines directly forms after oxygen-transfer, while nitroalkanes as main products for secondary and primary amines are yielded after a series of reactions mediated by hydroxylamine and nitrosoalkane with a ΔG≠ value of 10–13 kcal mol−1. Regarding the minor N-dealkylated products for all amines, alkylamino alcohol is an important intermediate possibly generated via a radical reaction pathway with a ΔG≠ value of 21–34 kcal mol−1. Additionally, comparison of the reactivity of aliphatic amines, hydroxylamines and alkylamino alcohols with ozone was made and elucidated in this study. The results are expected to expand our understanding of the degradation mechanisms for nitrogenous compounds during ozonation.
中文翻译:

臭氧化作用下简单脂肪胺的降解机理:DFT研究
脂肪胺作为溶解有机氮(DON)的常见成分,在臭氧化过程中表现出高反应活性;但是,我们对其降解机理的了解非常有限。在这项研究中,选择甲胺(MA)和乙胺(EA)以及它们的仲胺和叔胺(DMA,DEA,TMA和TEA)作为脂肪胺模型,并使用DFT方法研究了它们在臭氧氧化过程中的降解机理。最初和快速发生氧气转移反应在所有上述胺与一Δ臭氧化ģ ≠ 8-10千卡摩尔的值-1与10实验速率常数大协议4到10个7中号-1小号- 1。而且,N-氧化物是叔胺的主要降解产物,是在氧转移后直接形成的,而硝基烷是仲胺和伯胺的主要产物,是在羟胺和亚硝基烷烃介导的一系列反应后产生的,ΔG ≠ 10-13 kcal mol -1。关于所有胺的次要N-脱烷基化产物,烷基氨基醇是可能通过自由基反应途径生成的重要中间体,其ΔG ≠值为21–34 kcal mol -1。另外,在本研究中比较了脂肪族胺,羟胺和烷基氨基醇与臭氧的反应性。预期结果将扩大我们对臭氧化过程中含氮化合物降解机理的理解。
更新日期:2021-02-17
中文翻译:

臭氧化作用下简单脂肪胺的降解机理:DFT研究
脂肪胺作为溶解有机氮(DON)的常见成分,在臭氧化过程中表现出高反应活性;但是,我们对其降解机理的了解非常有限。在这项研究中,选择甲胺(MA)和乙胺(EA)以及它们的仲胺和叔胺(DMA,DEA,TMA和TEA)作为脂肪胺模型,并使用DFT方法研究了它们在臭氧氧化过程中的降解机理。最初和快速发生氧气转移反应在所有上述胺与一Δ臭氧化ģ ≠ 8-10千卡摩尔的值-1与10实验速率常数大协议4到10个7中号-1小号- 1。而且,N-氧化物是叔胺的主要降解产物,是在氧转移后直接形成的,而硝基烷是仲胺和伯胺的主要产物,是在羟胺和亚硝基烷烃介导的一系列反应后产生的,ΔG ≠ 10-13 kcal mol -1。关于所有胺的次要N-脱烷基化产物,烷基氨基醇是可能通过自由基反应途径生成的重要中间体,其ΔG ≠值为21–34 kcal mol -1。另外,在本研究中比较了脂肪族胺,羟胺和烷基氨基醇与臭氧的反应性。预期结果将扩大我们对臭氧化过程中含氮化合物降解机理的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号