当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycine-Assisted Hydrothermal Leaching of LiCoO2/LiNiO2 Cathode Materials with High Efficiency and Negligible Acid Corrosion Employing Batch and Continuous Flow System
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-02-16 , DOI: 10.1021/acssuschemeng.0c08703 Qingxin Zheng 1 , Kensuke Shibazaki 2 , Seiya Hirama 3 , Yuta Iwatate 2 , Atsushi Kishita 1 , Yuya Hiraga 1 , Yuta Nakayasu 4 , Masaru Watanabe 1, 5
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-02-16 , DOI: 10.1021/acssuschemeng.0c08703 Qingxin Zheng 1 , Kensuke Shibazaki 2 , Seiya Hirama 3 , Yuta Iwatate 2 , Atsushi Kishita 1 , Yuya Hiraga 1 , Yuta Nakayasu 4 , Masaru Watanabe 1, 5
Affiliation
|
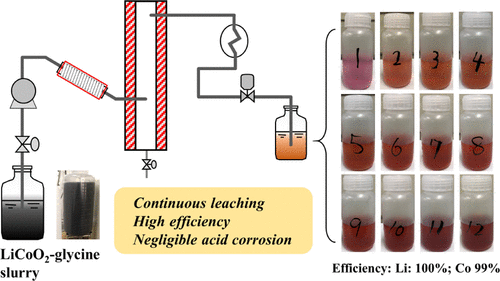
Glycine was applied as the leachant for the hydrothermal leaching of lithium-ion battery (LIB) cathode materials, LiCoO2 and LiNiO2, at 90–180 °C for 5–90 min. LiCoO2 was completely leached at 180 °C for 30 min. It was revealed that Co(III)–glycine complex formed first and was gradually reduced to Co(II)–glycine complex. Compared to LiCoO2, LiNiO2 required a lower temperature and shorter time for complete leaching. Different from using inorganic acids or organic acids like citric acid, pH values of the glycine solution before or after hydrothermal leaching of LiCoO2 or LiNiO2 was in the range 5.8–9.0, closer to neutral. In the mechanism analysis, the consumption of glycine was attributed to the oxidation of glycine and the formation reaction of Co–glycine complex; during the hydrothermal leaching at 180 °C for 30 min, more than half of leached Co was calculated to be reduced from trivalent to divalent. The shrinking unreacted core model was adopted to study the kinetics. As a result, hydrothermal leaching of Li and Co with glycine was well fitted by the interface reaction control, and the activation energies of Li and Co leaching were 87.5 and 72.2 kJ/mol, respectively. With the successful running of continuous hydrothermal leaching of LiCoO2 with glycine using a specifically customized flow system, LIB cathode materials were leached through the green and continuous process with a high efficiency close to 100%, and the acid corrosion was detected to be as light as to be almost negligible, for the first time.
中文翻译:

利用间歇和连续流系统,利用甘氨酸辅助的LiCoO 2 / LiNiO 2阴极材料的高效水热浸出,酸腐蚀可忽略不计
甘氨酸被用作锂离子电池(LIB)正极材料LiCoO 2和LiNiO 2的水热浸出液,在90–180°C下进行5–90分钟浸出。LiCoO 2在180°C下完全浸出30分钟。结果表明,Co(III)-甘氨酸复合物首先形成,然后逐渐还原为Co(II)-甘氨酸复合物。与LiCoO 2相比,LiNiO 2需要较低的温度和较短的时间来完成浸出。与使用无机酸或有机酸(如柠檬酸)不同,在LiCoO 2或LiNiO 2的水热浸出之前或之后,甘氨酸溶液的pH值在5.8-9.0范围内,接近中性。在机理分析中,甘氨酸的消耗归因于甘氨酸的氧化和Co-甘氨酸复合物的形成反应。在180°C水热浸出30分钟的过程中,超过一半的浸出Co被计算为从三价还原为二价。采用收缩的未反应核模型来研究动力学。结果,通过界面反应控制很好地拟合了Li和Co与甘氨酸的水热浸出,Li和Co的浸出活化能分别为87.5和72.2 kJ / mol。随着LiCoO 2连续水热浸出的成功运行 使用专门定制的流动系统使用甘氨酸,LIB阴极材料通过绿色连续工艺浸出,效率接近100%,并且首次检测到酸腐蚀几乎可以忽略不计。
更新日期:2021-03-01
中文翻译:

利用间歇和连续流系统,利用甘氨酸辅助的LiCoO 2 / LiNiO 2阴极材料的高效水热浸出,酸腐蚀可忽略不计
甘氨酸被用作锂离子电池(LIB)正极材料LiCoO 2和LiNiO 2的水热浸出液,在90–180°C下进行5–90分钟浸出。LiCoO 2在180°C下完全浸出30分钟。结果表明,Co(III)-甘氨酸复合物首先形成,然后逐渐还原为Co(II)-甘氨酸复合物。与LiCoO 2相比,LiNiO 2需要较低的温度和较短的时间来完成浸出。与使用无机酸或有机酸(如柠檬酸)不同,在LiCoO 2或LiNiO 2的水热浸出之前或之后,甘氨酸溶液的pH值在5.8-9.0范围内,接近中性。在机理分析中,甘氨酸的消耗归因于甘氨酸的氧化和Co-甘氨酸复合物的形成反应。在180°C水热浸出30分钟的过程中,超过一半的浸出Co被计算为从三价还原为二价。采用收缩的未反应核模型来研究动力学。结果,通过界面反应控制很好地拟合了Li和Co与甘氨酸的水热浸出,Li和Co的浸出活化能分别为87.5和72.2 kJ / mol。随着LiCoO 2连续水热浸出的成功运行 使用专门定制的流动系统使用甘氨酸,LIB阴极材料通过绿色连续工艺浸出,效率接近100%,并且首次检测到酸腐蚀几乎可以忽略不计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号