当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor-targeted gene therapy with lipid nanoparticles inhibits tumor-associated adipocytes and remodels the immunosuppressive tumor microenvironment in triple-negative breast cancer
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2021-2-15 , DOI: 10.1039/d0nh00588f Yun Liu 1 , Karthik Tiruthani , Menglin Wang , Xuefei Zhou , Nasha Qiu , Yang Xiong , Chad V Pecot , Rihe Liu , Leaf Huang
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2021-2-15 , DOI: 10.1039/d0nh00588f Yun Liu 1 , Karthik Tiruthani , Menglin Wang , Xuefei Zhou , Nasha Qiu , Yang Xiong , Chad V Pecot , Rihe Liu , Leaf Huang
Affiliation
|
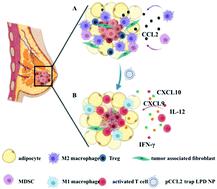
Adipocytes are the primary cellular components within the tumor microenvironment (TME) of triple-negative breast cancer (TNBC). Increasing evidence suggests that tumor-associated adipocytes (TAAs) can aggravate tumor progression, exacerbate the immunosuppressive TME and compromise therapeutic efficacy. In this study, the biological effect of TAAs within the breast cancer TME is first investigated, and the C–C Motif Chemokine Ligand 2 (CCL2) which is mainly secreted by TAAs in the extracellular environment is identified as the key mediator. CCL2 recruits immune cells such as monocytes and macrophages that further differentiated into immunosuppressive myeloid-derived suppressor cells (MDSCs) and M2 macrophages. To manipulate CCL2-mediated immune response, a protein trap that binds with CCL2 with high affinity and specificity is designed. The plasmid DNA encoding the CCL2 trap (pCCL2) is specifically delivered to the TME by using targeted lipid-protamine-DNA (LPD) nanoparticles to locally express the CCL2 trap and ameliorate the immunosuppressive TME. Significantly, compared with the commercially available CCL2 antibody, this strategy shows enhanced therapeutic efficacy and appreciable tumor growth inhibition. Furthermore, the pCCL2 trap treatment successfully suppresses TAAs, increases T cell infiltration and decreases the population of immunosuppressive M2 macrophages and MDSCs. Further studies show that the pCCL2 trap could facilitate PD-L1 blockade immunotherapy, demonstrating its translation potential.
中文翻译:

使用脂质纳米粒子进行肿瘤靶向基因治疗可抑制肿瘤相关脂肪细胞并重塑三阴性乳腺癌的免疫抑制肿瘤微环境
脂肪细胞是三阴性乳腺癌(TNBC)肿瘤微环境(TME)中的主要细胞成分。越来越多的证据表明,肿瘤相关脂肪细胞 (TAA) 会加剧肿瘤进展、加剧免疫抑制性 TME 并损害治疗效果。本研究首先研究了TAA在乳腺癌TME中的生物学效应,并确定细胞外环境中TAA主要分泌的C-C基序趋化因子配体2(CCL2)为关键介质。 CCL2 招募单核细胞和巨噬细胞等免疫细胞,这些细胞进一步分化为免疫抑制性骨髓源性抑制细胞 (MDSC) 和 M2 巨噬细胞。为了操纵 CCL2 介导的免疫反应,设计了一种以高亲和力和特异性与 CCL2 结合的蛋白质陷阱。通过使用靶向脂质-鱼精蛋白-DNA (LPD) 纳米粒子,将编码 CCL2 陷阱 (pCCL2) 的质粒 DNA 特异性递送至 TME,以局部表达 CCL2 陷阱并改善免疫抑制性 TME。值得注意的是,与市售的 CCL2 抗体相比,该策略显示出增强的治疗效果和明显的肿瘤生长抑制作用。此外,pCCL2 trap 处理成功地抑制了 TAA,增加了 T 细胞浸润并减少了免疫抑制性 M2 巨噬细胞和 MDSC 的数量。进一步的研究表明,pCCL2 陷阱可以促进 PD-L1 阻断免疫疗法,证明了其翻译潜力。
更新日期:2021-02-15
中文翻译:

使用脂质纳米粒子进行肿瘤靶向基因治疗可抑制肿瘤相关脂肪细胞并重塑三阴性乳腺癌的免疫抑制肿瘤微环境
脂肪细胞是三阴性乳腺癌(TNBC)肿瘤微环境(TME)中的主要细胞成分。越来越多的证据表明,肿瘤相关脂肪细胞 (TAA) 会加剧肿瘤进展、加剧免疫抑制性 TME 并损害治疗效果。本研究首先研究了TAA在乳腺癌TME中的生物学效应,并确定细胞外环境中TAA主要分泌的C-C基序趋化因子配体2(CCL2)为关键介质。 CCL2 招募单核细胞和巨噬细胞等免疫细胞,这些细胞进一步分化为免疫抑制性骨髓源性抑制细胞 (MDSC) 和 M2 巨噬细胞。为了操纵 CCL2 介导的免疫反应,设计了一种以高亲和力和特异性与 CCL2 结合的蛋白质陷阱。通过使用靶向脂质-鱼精蛋白-DNA (LPD) 纳米粒子,将编码 CCL2 陷阱 (pCCL2) 的质粒 DNA 特异性递送至 TME,以局部表达 CCL2 陷阱并改善免疫抑制性 TME。值得注意的是,与市售的 CCL2 抗体相比,该策略显示出增强的治疗效果和明显的肿瘤生长抑制作用。此外,pCCL2 trap 处理成功地抑制了 TAA,增加了 T 细胞浸润并减少了免疫抑制性 M2 巨噬细胞和 MDSC 的数量。进一步的研究表明,pCCL2 陷阱可以促进 PD-L1 阻断免疫疗法,证明了其翻译潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号