当前位置:
X-MOL 学术
›
Chem. Data Collect.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Wadsworth Emmons cyclopropanation mediated diastereoselective Syntheses of α-epoxy- trans-disubstituted cyclopropane and α-hydroxy-trans-disubstituted cyclopropane
Chemical Data Collections Pub Date : 2021-02-15 , DOI: 10.1016/j.cdc.2021.100667 Ramakrishna D.S.
中文翻译:

Wadsworth Emmons环丙烷介导的α-环氧-反式-二取代环丙烷和α-羟基-反式-二取代环丙烷的非对映选择性合成
更新日期:2021-02-26
Chemical Data Collections Pub Date : 2021-02-15 , DOI: 10.1016/j.cdc.2021.100667 Ramakrishna D.S.
|
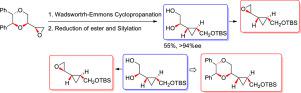
The present work describes the diastereoselective Syntheses of α-epoxy-trans-disubstituted cyclopropane and α-hydroxy-trans-disubstitutedcyclopropane. The genesis of chirality in the targeted products is based on the exploitation of catalytic asymmetric transfer hydrogenation (CATHy) reaction. Asymmetric Cyclopropanation was carried out using Wadsworth–Emmons protocol. Among the surveyed conditions, employing TEPA, epoxide, NaH in toluene under reflux furnished the desired cyclopropane with 85% yield.
中文翻译:

Wadsworth Emmons环丙烷介导的α-环氧-反式-二取代环丙烷和α-羟基-反式-二取代环丙烷的非对映选择性合成
本工作描述了α-环氧-反式-二取代的环丙烷和α-羟基-反式-二取代的环丙烷的非对映选择性合成。目标产物中手性的产生是基于对催化不对称转移氢化(CATHy)反应的利用。使用Wadsworth–Emmons方案进行不对称环丙烷化。在所考察的条件中,使用TEPA,环氧化物,NaH在甲苯中回流,可提供所需的环丙烷,产率为85%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号