Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2021-02-14 , DOI: 10.1016/j.saa.2021.119572 Faez Iqbal Khan 1 , Honghong Song 2 , Fakhrul Hassan 2 , Jing Tian 2 , Lixia Tang 2 , Dakun Lai 1 , Feng Juan 2
|
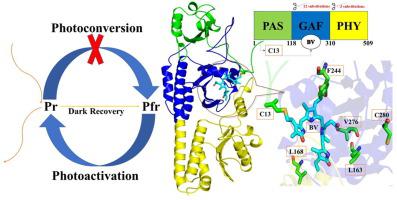
A photoactivatable near-infrared fluorescent protein (NIR-FP) PAiRFP1 has been developed by 15 amino acid substitutions in its nonfluorescent template Agp2. In our previous communication, we investigated the role of three amino acids in PHY domain distal from BV molecule. The impact of the twelve amino acids in GAF domain, especially five residues near BV-binding pocket is unclear. In this paper, PCR based reverse mutagenesis, spectroscopic methods, molecular modelling and simulations have been employed to explore the roles of these substitutions during the molecular evolution of PAiRFP1. It was found that the residue L163 is important for protein folding in PAiRFP1. The residues F244 and C280 exerted remarkable effects on molar extinction coefficient, NIR fluorescence quantum yield, molecular brightness, fluorescence fold, and dark recovery rate. The residues F244 and V276 modulate the maximum absorption and emission peak position. The reverse mutant L168M exhibited a higher fluorescence fold than PAiRFP1. Additionally, the reverse mutants V203A, V294E, S218G and D127G possessed better spectral properties than PAiRFP1. This study is important for the rational design of a better BphP-based photoactivatable NIR-FPs.
中文翻译:

氨基酸取代对光活化近红外荧光蛋白 PAiRFP1 行为的影响
一种可光活化的近红外荧光蛋白 (NIR-FP) PAiRFP1 已通过其非荧光模板 Agp2 中的 15 个氨基酸取代而开发。在我们之前的交流中,我们研究了三种氨基酸在远离 BV 分子的 PHY 结构域中的作用。GAF 结构域中 12 个氨基酸的影响,尤其是 BV 结合口袋附近的 5 个残基,其影响尚不清楚。本文采用基于 PCR 的反向诱变、光谱方法、分子建模和模拟来探索这些取代在 PAiRFP1 分子进化过程中的作用。发现残基 L163 对 PAiRFP1 中的蛋白质折叠很重要。残基F244和C280对摩尔消光系数、近红外荧光量子产率、分子亮度、荧光倍数和暗恢复率有显着影响。残基 F244 和 V276 调节最大吸收和发射峰位置。反向突变体 L168M 表现出比 PAiRFP1 更高的荧光倍数。此外,反向突变体 V203A、V294E、S218G 和 D127G 具有比 PAiRFP1 更好的光谱特性。这项研究对于合理设计更好的基于 BphP 的可光激活 NIR-FP 具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号