Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-02-14 , DOI: 10.1016/j.bmc.2021.116056 Ryo Mizojiri 1 , Daisuke Tomita 1 , Masako Sasaki 1 , Yoshihiko Satoh 1 , Yukiko Yamamoto 1 , Hiroyuki Sumi 1 , Hironobu Maezaki 1
|
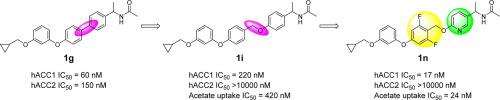
A structure–activity relationship (SAR) study towards novel ACC1-selective inhibitors was carried out by modifying the molecular length of the linker in biaryl derivative 1 g, an ACC1/2 dual inhibitor. Ultimately, this leads us to discover novel phenoxybenzyloxy derivative 1i as a potent ACC1-selective inhibitor. Further chemical modification of this scaffold to improve cellular potency as well as physicochemical and pharmacokinetic (PK) properties produced N-2-(pyridin-2-ylethyl)acetamide derivative 1n, which showed highly potent ACC1-selective inhibition as well as sufficient PK profile for further in vivo evaluations. Oral administration of 1n significantly reduced the concentration of malonyl-CoA in HCT-116 xenograft tumors at doses of 100 mg/kg. Accordingly, our novel series of potent ACC1-selective inhibitors represents a set of useful orally-available research tools, as well as potential therapeutic agents for cancer and fatty acid-related diseases.
中文翻译:

通过联苯ACC1/2双重抑制剂的化学修饰设计和合成作为选择性ACC1抑制剂的单环衍生物
通过改变ACC1/2 双重抑制剂联芳基衍生物1 g 中连接子的分子长度,对新型 ACC1 选择性抑制剂进行了构效关系 (SAR) 研究。最终,这使我们发现了新型苯氧基苄氧基衍生物1i作为有效的 ACC1 选择性抑制剂。对该支架的进一步化学修饰以提高细胞效力以及物理化学和药代动力学 (PK) 特性,产生了N -2-(吡啶-2-基乙基)乙酰胺衍生物1n,其显示出高效的 ACC1 选择性抑制以及足够的 PK 特征用于进一步的体内评估。口服1n以 100 mg/kg 的剂量显着降低 HCT-116 异种移植肿瘤中丙二酰辅酶 A 的浓度。因此,我们的新型强效 ACC1 选择性抑制剂系列代表了一组有用的口服研究工具,以及癌症和脂肪酸相关疾病的潜在治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号