当前位置:
X-MOL 学术
›
Adv. Theory Simul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Intra‐Molecular Proton Transfer of Octahydrotriborate and Exploring the Dehydrogenation Pathways of NH4B3H8 by DFT Calculations
Advanced Theory and Simulations ( IF 2.9 ) Pub Date : 2021-02-11 , DOI: 10.1002/adts.202000287 Peng Gao 1 , Jie Zhang 2, 3
Advanced Theory and Simulations ( IF 2.9 ) Pub Date : 2021-02-11 , DOI: 10.1002/adts.202000287 Peng Gao 1 , Jie Zhang 2, 3
Affiliation
|
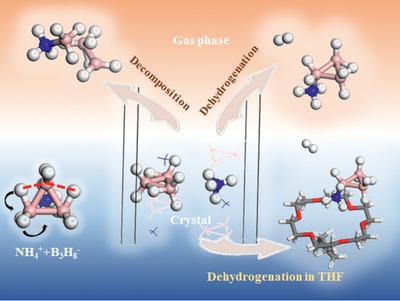
The intra‐molecular proton transfer of octahydrotriborate, [B3H8]−, is discovered by density functional theory (DFT) computational studies; such a transfer can largely impact its dehydrogenation pathways. The DFT calculation results further disclose the generation of another triborane intermediate product, B3H9, which is formed via inter‐molecular proton transfer from [NH4]+ to [B3H8]−. Additionally, this intermediate product is unstable and can release hydrogen easily, as the corresponding energy barrier via this pathway is only 12.0 kcal mol−1. Such a relative energy is much lower than that of the routinely raised pathway, which mainly depends on the dihydrogen interaction between the and . This new mechanism is able to explain several experimental observations involving the dehydrogenation of NH4B3H8. Moreover, the detailed dehydrogenations of NH4B3H8 in different states are also studied, and the role of chemical environment during dehydrogenation is demonstrated.
中文翻译:

通过DFT计算了解八氢三硼酸盐的分子内质子转移并探索NH4B3H8的脱氢途径
八氢三硼酸盐[B 3 H 8 ] -的分子内质子转移是通过密度泛函理论(DFT)的计算研究发现的。这种转移可大大影响其脱氢途径。DFT计算结果进一步揭示了另一种三硼烷中间产物B 3 H 9的生成,该产物是通过分子间质子从[NH 4 ] +转移至[B 3 H 8 ] -形成的。另外,该中间产物不稳定并且容易释放氢,因为通过该途径的相应能量屏障仅为12.0 kcal mol -1。这样的相对能量比常规升高的途径的能量要低得多,后者主要取决于氢原子之间的二氢相互作用。 和 。这种新机制能够解释涉及NH 4 B 3 H 8脱氢的一些实验观察。此外,还研究了NH 4 B 3 H 8在不同状态下的详细脱氢作用,并证明了化学环境在脱氢过程中的作用。
更新日期:2021-03-09
中文翻译:

通过DFT计算了解八氢三硼酸盐的分子内质子转移并探索NH4B3H8的脱氢途径
八氢三硼酸盐[B 3 H 8 ] -的分子内质子转移是通过密度泛函理论(DFT)的计算研究发现的。这种转移可大大影响其脱氢途径。DFT计算结果进一步揭示了另一种三硼烷中间产物B 3 H 9的生成,该产物是通过分子间质子从[NH 4 ] +转移至[B 3 H 8 ] -形成的。另外,该中间产物不稳定并且容易释放氢,因为通过该途径的相应能量屏障仅为12.0 kcal mol -1。这样的相对能量比常规升高的途径的能量要低得多,后者主要取决于氢原子之间的二氢相互作用。 和 。这种新机制能够解释涉及NH 4 B 3 H 8脱氢的一些实验观察。此外,还研究了NH 4 B 3 H 8在不同状态下的详细脱氢作用,并证明了化学环境在脱氢过程中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号