当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Late‐stage Rh(II)‐catalyzed Nitrene Transfer for the Synthesis of Guaianolide Analogs with Enhanced Antiproliferative Activity
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-02-12 , DOI: 10.1002/ejoc.202100074 Sebastián J. Castro 1 , José M. Padrón 2 , Benjamin Darses 3 , Viviana E. Nicotra 1 , Philippe Dauban 4
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-02-12 , DOI: 10.1002/ejoc.202100074 Sebastián J. Castro 1 , José M. Padrón 2 , Benjamin Darses 3 , Viviana E. Nicotra 1 , Philippe Dauban 4
Affiliation
|
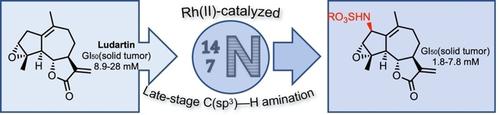
Application of rhodium(II)‐catalyzed intermolecular nitrene transfers to naturally‐occurring guaianolide compounds led to isolating nine new derivatives that possess an amino functional group at C‐1, C‐2, C‐10, C‐11, and/or C‐13 positions. The relevance of a late‐stage C−H amination strategy in medicinal chemistry is demonstrated by the improved antiproliferative activity of the C‐2 aminated analogs of guaianolide products.

中文翻译:

晚期Rh(II)催化的丁二烯转移,用于合成具有增强抗增殖活性的瓜亚胺酯类似物
铑(II)催化的分子间氮烯转移到天然存在的愈创木酚内酯化合物上导致分离出9个在C-1,C-2,C-10,C-11和/或C处具有氨基官能团的新衍生物‐13个职位。新型C–H胺化策略在药物化学中的相关性由愈创木酚内酯产品的C–2胺化类似物的抗增殖活性提高证明。

更新日期:2021-03-23

中文翻译:

晚期Rh(II)催化的丁二烯转移,用于合成具有增强抗增殖活性的瓜亚胺酯类似物
铑(II)催化的分子间氮烯转移到天然存在的愈创木酚内酯化合物上导致分离出9个在C-1,C-2,C-10,C-11和/或C处具有氨基官能团的新衍生物‐13个职位。新型C–H胺化策略在药物化学中的相关性由愈创木酚内酯产品的C–2胺化类似物的抗增殖活性提高证明。












































 京公网安备 11010802027423号
京公网安备 11010802027423号