Progress in Natural Science: Materials International ( IF 4.8 ) Pub Date : 2021-02-11 , DOI: 10.1016/j.pnsc.2021.01.008 Yong Wu , Yuanting Peng , Xiaojing Jiang , Hui Zeng , Zeyuan Wang , Jie Zheng , Xingguo Li
|
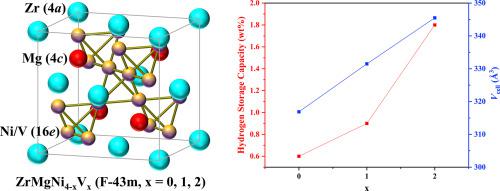
The development of hydrogen energy is hindered by the lack of high-efficiency hydrogen storage materials. To explore new high-capacity hydrogen storage alloys, reversible hydrogen storage in AB2-type alloy is realized by using A or B-side elemental substitution. The substitution of small atomic-radius element Zr and Mg on A-side of YNi2 and partial substitution of large atomic-radius element V on B-side of YNi2 alloy was investigated in this study. The obtained ZrMgNi4, ZrMgNi3V, and ZrMgNi2V2 alloys remained single Laves phase structure at as-annealed, hydrogenated and dehydrogenated states, indicating that the hydrogen-induced amorphization and disproportionation was eliminated. From ZrMgNi4 to ZrMgNi2V2 with the increase of the degree of vanadium substitution, the reversible hydrogen storage capacity increased from 0.6 wt% (0.35H/M) to 1.8 wt% (1.0H/M), meanwhile the lattice stability gradually increased. The ZrMgNi2V2 alloy could absorb 1.8 wt% hydrogen in about 2 h at 300 K under 4 MPa H2 pressure and reversibly desorb the absorbed hydrogen in approximately 30 min at 473 K without complicated activation process. The prominent properties of ZrMgNi2V2 elucidate its high potential for hydrogen storage application.
中文翻译:

AB 2型Zr–Mg–Ni–V基储氢合金的可逆氢化
缺乏高效的储氢材料阻碍了氢能的发展。为了探索新的高容量储氢合金,通过使用A或B侧元素取代实现AB 2型合金中的可逆储氢。小原子半径元件Zr和Mg对YNI的A侧置换2和大的原子半径单元V的部分取代上YNI的B侧2合金在本研究中进行了研究。所获得的ZrMgNi 4,ZrMgNi 3 V和ZrMgNi 2 V 2合金在退火,氢化和脱氢状态下保持单一的Laves相结构,表明消除了氢诱导的非晶化和歧化。从ZrMgNi 4到ZrMgNi 2 V 2随着钒取代度的增加,可逆储氢容量从0.6重量%(0.35H / M)增加到1.8重量%(1.0H / M),同时晶格稳定性逐渐提高。ZrMgNi 2 V 2合金可在4 MPa H 2压力下于300 K下约2 h吸收1.8 wt%的氢,并在473 K下约30 min可逆地解吸所吸收的氢,而无需复杂的活化过程。ZrMgNi的突出特性2 V 2阐明了其在储氢应用中的高潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号