Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2021-02-11 , DOI: 10.1016/j.chemphyslip.2021.105060 Kushagra Khanna 1 , Nitin Sharma 2 , Sonalika Rawat 3 , Nazia Khan 4 , Ritu Karwasra 3 , Nazeer Hasan 4 , Abhishek Kumar 3 , Gaurav Kumar Jain 5 , Dhruv Kumar Nishad 3 , Sakshum Khanna 6 , Harvinder Popli 5 , Aseem Bhatnagar 3
|
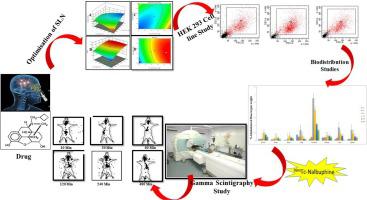
Pain is a noxious stimulus caused due to tissue damage and varies from mild to severe. Nalbuphine (NLB) is an approved, inexpensive, non-controlled, opioid agonist/antagonist analgesic used worldwide in various clinical settings for pain management. The current study aims to formulate NLB loaded solid lipid nanoparticles (SLNs) using solvent injection technology. The morphological and chemical structure of the developed SLNs were characterized using Field Emission Scanning Electron Microscopy (FESEM), Transmission Electron Microscopy (TEM) and Fourier Transformation Infrared Spectroscopy (FTIR). The results revealed from the point prediction confirmation in design expert software was the formulation of NLB-SLNs with an average particle size of (170.07 ± 25.1 nm), encapsulation efficiency (93.6 ± 1.5%) & loading capacity of 26.67%. The in-vitro permeation of developed NLB-SLNs was observed to be 94.18% at 8 h when compared with NLB solution whose maximum permeation was seen within 3 h of application. Efficacy of the formulation was also evaluated using eddy's hot plate method, where the onset of action started within 10 min of administration, and the maximum effect was observed at 1 h. The NLB-SLNs was screened for cytotoxicity in human embryonic kidney cells (HEK-293), and the dosage was considered safe when administered intranasally in animal since no detectable effect to the brain was observed. Biodistribution and gamma scintigraphy study of NLB-SLNs showed the prepared formulation reaching the target site, i.e. brain and was retained. Conclusively, the prepared NLB-SLNs formulation was safe and effective in producing an analgesic effect in vivo.
中文翻译:

用于治疗疼痛的鼻内固体脂质纳米颗粒:全因子设计方法、表征和伽玛闪烁扫描
疼痛是由组织损伤引起的有害刺激,从轻微到严重不等。纳布啡 (NLB) 是一种经批准的、廉价的、非受控的阿片类激动剂/拮抗剂镇痛剂,在全球范围内用于各种临床环境中的疼痛管理。目前的研究旨在使用溶剂注射技术配制负载 NLB 的固体脂质纳米颗粒 (SLN)。使用场发射扫描电子显微镜 (FESEM)、透射电子显微镜 (TEM) 和傅立叶变换红外光谱 (FTIR) 对开发的 SLN 的形态和化学结构进行了表征。设计专家软件的点预测确认结果显示,NLB-SLNs的配方平均粒径为(170.07±25.1 nm),包封率(93.6±1.5%)和负载能力为26.67%。这与 NLB 溶液相比,已开发的 NLB-SLN 在 8 小时时的体外渗透率为 94.18%,而 NLB 溶液在应用后 3 小时内达到最大渗透率。还使用涡流热板法评估了制剂的功效,其中在给药后 10 分钟内开始起效,并在 1 小时时观察到最大效果。NLB-SLNs 在人胚胎肾细胞 (HEK-293) 中进行了细胞毒性筛选,当在动物中鼻内给药时,该剂量被认为是安全的,因为没有观察到对大脑的可检测影响。NLB-SLN 的生物分布和伽马闪烁扫描研究表明,制备的制剂到达目标部位,即大脑并被保留。综上所述,制备的 NLB-SLNs 制剂在产生镇痛作用方面是安全有效的。体内。










































 京公网安备 11010802027423号
京公网安备 11010802027423号