当前位置:
X-MOL 学术
›
Immunol. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adhesion to E-selectin primes macrophages for activation through AKT and mTOR
Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2021-02-10 , DOI: 10.1111/imcb.12447 Julie M Davies 1 , Kristen J Radford 1 , Jakob Begun 1 , Jean-Pierre Levesque 1 , Ingrid G Winkler 1
Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2021-02-10 , DOI: 10.1111/imcb.12447 Julie M Davies 1 , Kristen J Radford 1 , Jakob Begun 1 , Jean-Pierre Levesque 1 , Ingrid G Winkler 1
Affiliation
|
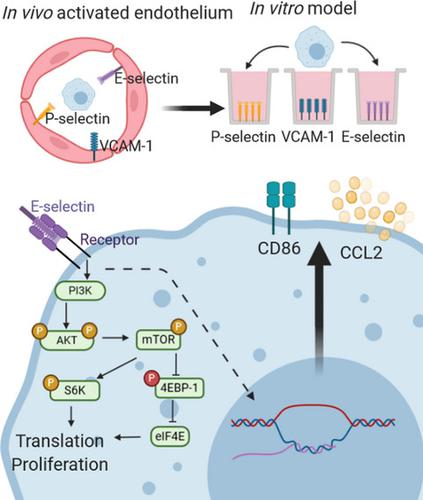
The endothelial adhesion protein E-selectin/CD62E is not required for leukocyte homing, unlike closely related family member P-selectin/CD62P. As transmigration through the endothelium is one of the first steps in generating a local immune response, we hypothesized that E-selectin may play additional roles in the early stages of immune activation. We found contact with E-selectin, but not P-selectin or vascular cell adhesion molecule 1 (CD106), induced phosphorylation of protein kinase B (AKT) and nuclear factor-κB in mouse bone marrow-derived macrophages (BMDMs) in vitro. This occurred within 15 min of E-selectin contact and was dependent on phosphatidylinositol-3 kinase activity. Binding to E-selectin activated downstream proteins including mammalian target of rapamycin, p70 ribosomal protein S6 kinase and eukaryotic translation initiation factor 4E-binding protein 1. Functionally, adhesion to E-selectin induced upregulation of CD86 expression and CCL2 secretion. We next asked whether contact with E-selectin impacts further BMDM stimulation. We found enhanced secretion of both interleukin (IL)-10 and CCL2, but not tumor necrosis factor or IL-6 in response to lipopolysaccharide (LPS) stimulation after adhesion to E-selectin. Importantly, adhesion to E-selectin did not polarize BMDMs to one type of response but enhanced both arginase activity and nitric oxide production following IL-4 or LPS stimulation, respectively. In cultured human monocytes, adhesion to E-selectin similarly induced phosphorylation of AKT. Finally, when E-selectin was blocked in vivo in mice, thioglycollate-elicited macrophages showed reduced CD86 expression, validating our in vitro studies. Our results imply functions for E-selectin beyond homing and suggest that E-selectin plays an early role in priming and amplifying innate immune responses.
中文翻译:

与 E-选择素的粘附使巨噬细胞通过 AKT 和 mTOR 激活
与密切相关的家族成员 P-选择素/CD62P 不同,白细胞归巢不需要内皮粘附蛋白 E-选择素/CD62E。由于通过内皮的迁移是产生局部免疫反应的第一步,我们假设 E-选择素可能在免疫激活的早期阶段发挥额外的作用。我们发现与 E-选择素接触,但不是 P-选择素或血管细胞粘附分子 1 (CD106),在体外诱导小鼠骨髓衍生巨噬细胞 (BMDM) 中蛋白激酶 B (AKT) 和核因子-κB 的磷酸化. 这发生在 E-选择素接触的 15 分钟内,并且取决于 phosphatidylinositol-3 激酶活性。与 E-selectin 结合激活下游蛋白,包括哺乳动物雷帕霉素靶蛋白、p70 核糖体蛋白 S6 激酶和真核翻译起始因子 4E 结合蛋白 1。在功能上,与 E-selectin 的粘附诱导 CD86 表达和 CCL2 分泌的上调。我们接下来询问与 E-选择素的接触是否会影响进一步的 BMDM 刺激。我们发现白细胞介素 (IL)-10 和 CCL2 的分泌增加,但肿瘤坏死因子或 IL-6 对 E-选择素粘附后的脂多糖 (LPS) 刺激没有反应。重要的是,与 E-选择素的粘附不会使 BMDM 极化为一种类型的反应,但会在 IL-4 或 LPS 刺激后增强精氨酸酶活性和一氧化氮产生,分别。在培养的人单核细胞中,对 E-选择素的粘附同样诱导了 AKT 的磷酸化。最后,当 E-选择素被阻断时在小鼠体内,巯基乙酸诱导的巨噬细胞显示 CD86 表达降低,验证了我们的体外研究。我们的结果暗示 E-选择素的功能超出了归巢,并表明 E-选择素在引发和放大先天免疫反应中起早期作用。
更新日期:2021-02-10
中文翻译:

与 E-选择素的粘附使巨噬细胞通过 AKT 和 mTOR 激活
与密切相关的家族成员 P-选择素/CD62P 不同,白细胞归巢不需要内皮粘附蛋白 E-选择素/CD62E。由于通过内皮的迁移是产生局部免疫反应的第一步,我们假设 E-选择素可能在免疫激活的早期阶段发挥额外的作用。我们发现与 E-选择素接触,但不是 P-选择素或血管细胞粘附分子 1 (CD106),在体外诱导小鼠骨髓衍生巨噬细胞 (BMDM) 中蛋白激酶 B (AKT) 和核因子-κB 的磷酸化. 这发生在 E-选择素接触的 15 分钟内,并且取决于 phosphatidylinositol-3 激酶活性。与 E-selectin 结合激活下游蛋白,包括哺乳动物雷帕霉素靶蛋白、p70 核糖体蛋白 S6 激酶和真核翻译起始因子 4E 结合蛋白 1。在功能上,与 E-selectin 的粘附诱导 CD86 表达和 CCL2 分泌的上调。我们接下来询问与 E-选择素的接触是否会影响进一步的 BMDM 刺激。我们发现白细胞介素 (IL)-10 和 CCL2 的分泌增加,但肿瘤坏死因子或 IL-6 对 E-选择素粘附后的脂多糖 (LPS) 刺激没有反应。重要的是,与 E-选择素的粘附不会使 BMDM 极化为一种类型的反应,但会在 IL-4 或 LPS 刺激后增强精氨酸酶活性和一氧化氮产生,分别。在培养的人单核细胞中,对 E-选择素的粘附同样诱导了 AKT 的磷酸化。最后,当 E-选择素被阻断时在小鼠体内,巯基乙酸诱导的巨噬细胞显示 CD86 表达降低,验证了我们的体外研究。我们的结果暗示 E-选择素的功能超出了归巢,并表明 E-选择素在引发和放大先天免疫反应中起早期作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号