Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2021-02-09 , DOI: 10.1016/j.saa.2021.119570 Heba Abd El-Aziz , M.E. Fathy , M.M. Tolba , N. El-Enany , F.A. Aly
|
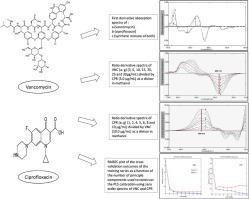
Four simple, rapid, accurate and precise spectrophotometric methods were established and validated in accordance with ICH Q2 (R1) guidelines for the simultaneous determination of Vancomycin (VNC) and Ciprofloxacin (CPR) in their raw materials, laboratory prepared mixtures and pharmaceutics. Method A depends on using first derivative spectrophotometry (D1) where VNC and CPR were resolved at 243.6 and 262.0 nm, respectively. Concerning method B, it is based on utilizing first derivative of ratio spectra (DD1) where determination was performed at the peak maxima at 244.0 nm and 258.0 nm for VNC and CPR, respectively. Two chemometric models were applied for the quantitative analysis of both drugs in their laboratory prepared mixtures, namely, partial least squares (PLS) (method C) and artificial neural network (ANN) (method D). For univariate methods linearity range for both drugs was in the range of 3–30 and 1–10 μg/mL for VNC and CPR, respectively. Multivariate calibration methods using five level, two factor calibration model for the development of 25 mixtures were also applied for the simultaneous estimation of the two drugs in their laboratory prepared mixture using spectral region from 200.0 to 300.0 nm using interval 1 nm. The suggested methods have been successfully extended to the assay of the two studied drugs in laboratory-prepared mixtures and pharmaceuticals with excellent recovery. First derivative spectrophotometry (D1) was also applied for the assay of both analytes in spiked human plasma with good recovery. No interaction with common pharmaceutical additives has been observed which indicate the selectivity of the method. The results obtained were favourably compared with those obtained applying the reported methods. The methods are validated in compliance with the ICH Q2 (R1) guidelines and the measured accuracy and precision are assessed to be within the accepted limits.
中文翻译:

用于同时评估万古霉素和环丙沙星实验室制备混合物中某些单变量和多变量分光光度法的研究及其在生物流体中的应用
根据ICH Q2(R1)指南建立并验证了四种简单,快速,准确和精确的分光光度法,以同时测定其原材料,实验室制备的混合物和药物中的万古霉素(VNC)和环丙沙星(CPR)。方法A取决于使用一阶导数分光光度法(D 1),其中VNC和CPR分别在243.6和262.0 nm处分辨。关于方法B,它基于比率光谱的一阶导数(DD 1),分别在VNC和CPR的244.0 nm和258.0 nm的最大峰值处进行测定。两种化学计量模型分别用于两种药物在实验室制备的混合物中的定量分析,分别是偏最小二乘(PLS)(方法C)和人工神经网络(ANN)(方法D)。对于单变量方法,VNC和CPR两种药物的线性范围分别为3–30和1–10μg/ mL。使用五级,两因素校准模型开发25种混合物的多变量校准方法还用于同时估计其实验室制备的混合物中两种药物的使用,光谱范围为200.0至300.0 nm,间隔为1 nm。所建议的方法已成功地扩展到实验室制备的混合物和药物中两种研究药物的测定,并具有优异的回收率。一阶导数分光光度法(D1)还用于加标人血浆中两种分析物的分析,回收率良好。没有观察到与普通药物添加剂的相互作用,表明该方法的选择性。将获得的结果与采用报道的方法获得的结果进行了比较。这些方法已按照ICH Q2(R1)指南进行了验证,并且测得的准确性和精密度被评估为在可接受的范围内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号