Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2021-02-09 , DOI: 10.1016/j.jmgm.2021.107864 Mahnaz Shojapour 1 , Faezeh Fatemi 2 , Somayeh Farahmand 1 , Marzieh Dehghan Shasaltaneh 3
|
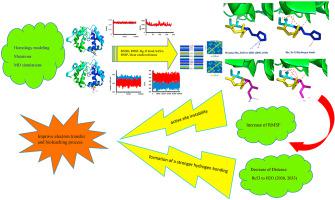
Acidithiobacillus ferrooxidans (Af) is an acidophilic bacterium that grows in rigid surroundings and gets its own energy from the oxidation of Fe2+ to Fe3+. These bacteria are involved in the bioleaching process. Cyc1 is a periplasmic protein with a crucial role in electron transportation in the respiratory chain. His53 of the Cyc1 protein, involved in electron transfer to CoxB, was selected for mutation and bioinformatics studies. His53 was substituted by Ile using PyMol software. Molecular dynamics simulations were performed for wild and mutant types of Cyc1 protein. The conformational changes of mutated protein were studied by analyzing RMSD, RMSF, SASA, Rg, H Bond, and DSSP. The results of the RMSF analysis indicated an increase in the flexibility of the ligand in the mutant. Finally, active site instability leads to an increase in the value of E0 at the mutation point and improving electron transfer. On the other, His53 in Cyc1 is interconnected to Glu126 in CoxB through the water molecule (W76) and hydrogen bonding. In the H53I mutation, there was a decrease in the distance between H2O 2030, 2033, and isoleucine 53, and subsequently, the distance to the water molecule 76 between the two proteins was reduced and strengthens the hydrogen bond between Cyc1 and CoxB, finally improves electron transfer and the bioleaching process.
中文翻译:

使用计算机方法研究H53I突变后Cyc 1蛋白质结构稳定性的方法,以提高氧化还原电位
嗜酸铁硫杆菌(Af)是一种嗜酸细菌,其在坚硬的环境中生长,并从Fe 2+氧化为Fe 3+获得自己的能量。这些细菌参与了生物浸出过程。Cyc 1是一种周质蛋白,在呼吸链中的电子运输中起着至关重要的作用。参与电子转移到CoxB的Cyc 1蛋白的His53被选择用于突变和生物信息学研究。使用PyMol软件将His53替换为Ile。对野生型和突变型Cyc 1进行了分子动力学模拟蛋白质。通过分析RMSD,RMSF,SASA,Rg,H键和DSSP,研究了突变蛋白的构象变化。RMSF分析的结果表明突变体中配体的柔性增加。最后,活性位点的不稳定性导致突变点的E 0值增加,并改善了电子传递。另一方面,Cyc 1中的His53通过水分子(W76)和氢键与CoxB中的Glu126互连。在H53I突变中,H2O 2030、2033与异亮氨酸53之间的距离减小,随后,两种蛋白质之间与水分子76的距离减小,并增强了Cyc 1之间的氢键 和CoxB,最终改善了电子转移和生物浸出过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号