当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effect of structural modification of antimicrobial peptides on their antimicrobial activity, hemolytic activity, and plasma stability
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-02-07 , DOI: 10.1002/psc.3306 Taoran Wang 1 , Cunbin Zou 1 , Na Wen 1 , Xingdong Liu 1 , Zhao Meng 1 , Siliang Feng 1 , Zhibing Zheng 1 , Qingbin Meng 1, 2 , Chenhong Wang 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-02-07 , DOI: 10.1002/psc.3306 Taoran Wang 1 , Cunbin Zou 1 , Na Wen 1 , Xingdong Liu 1 , Zhao Meng 1 , Siliang Feng 1 , Zhibing Zheng 1 , Qingbin Meng 1, 2 , Chenhong Wang 1
Affiliation
|
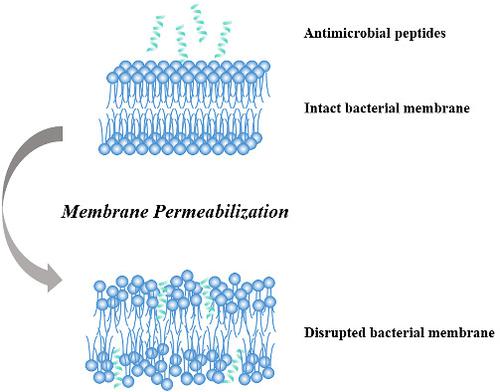
In this article, a series of modifications were made on an antimicrobial peptide F2,5,12W, including altering the amino acid sequence, introducing cysteine and other typical amino acids, developing peptide dimers via disulfide bonds, and conjugating with mPEG, in order to enhance the antimicrobial activity, plasma stability, and reduce the hemolytic activity of peptides. The results showed that mPEG conjugation could significantly improve the plasma stability and reduce the hemolytic activity of peptides, while the antimicrobial activity decreased meanwhile. However, altering the sequence of the peptide without changing its amino acid composition had little impact on its antimicrobial activity and plasma stability. The introduction of cysteine enhanced the plasma stability of peptides conspicuously, but at the same time, the increased hydrophobicity of peptides increased their hemolysis. The antimicrobial mechanism and cytotoxicity of the peptides with relatively high antimicrobial activity were also studied. In general, this study provided some ideas for the rational design and structure optimization of antimicrobial peptides.
中文翻译:

抗菌肽结构修饰对其抗菌活性、溶血活性和血浆稳定性的影响
在本文中,对抗菌肽 F 2,5,12进行了一系列修饰W,包括改变氨基酸序列,引入半胱氨酸等典型氨基酸,通过二硫键形成肽二聚体,与mPEG结合,以提高肽的抗菌活性、血浆稳定性和降低溶血活性。结果表明,mPEG结合能显着提高血浆稳定性,降低肽的溶血活性,同时抗菌活性降低。然而,改变肽的序列而不改变其氨基酸组成对其抗菌活性和血浆稳定性几乎没有影响。半胱氨酸的引入显着增强了肽的血浆稳定性,但同时,肽疏水性的增加也增加了它们的溶血。还研究了具有较高抗菌活性的肽的抗菌机制和细胞毒性。总的来说,本研究为抗菌肽的合理设计和结构优化提供了一些思路。
更新日期:2021-04-05
中文翻译:

抗菌肽结构修饰对其抗菌活性、溶血活性和血浆稳定性的影响
在本文中,对抗菌肽 F 2,5,12进行了一系列修饰W,包括改变氨基酸序列,引入半胱氨酸等典型氨基酸,通过二硫键形成肽二聚体,与mPEG结合,以提高肽的抗菌活性、血浆稳定性和降低溶血活性。结果表明,mPEG结合能显着提高血浆稳定性,降低肽的溶血活性,同时抗菌活性降低。然而,改变肽的序列而不改变其氨基酸组成对其抗菌活性和血浆稳定性几乎没有影响。半胱氨酸的引入显着增强了肽的血浆稳定性,但同时,肽疏水性的增加也增加了它们的溶血。还研究了具有较高抗菌活性的肽的抗菌机制和细胞毒性。总的来说,本研究为抗菌肽的合理设计和结构优化提供了一些思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号