Energetic Materials Frontiers Pub Date : 2020-09-08 , DOI: 10.1016/j.enmf.2020.09.001 Yu Zhang , Zishuai Xu , Luyao Zhang , Guangyuan Zhang , Dan He , Xiaolong Wang , Jian Ruan , Delong Zhang , Guoliang Jin , Xiaoping Ma , Xiaoliang He , Yanming Xu , Ying He , Jun Luo
|
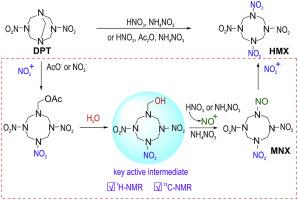
The problem whether 1-hydroxylmethyl-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane is the key active intermediate for the synthesis of 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane (HMX) from urotropine or 3,7-dinitro-1,3,5,7-tetraazabicyclo[3.3.1]nonane (DPT) remains to be illuminated for decades. In this paper, the hydrolysis and nitrolysis of the esterified intermediate 1-acetoxymethyl-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane (PHX) were studied to solve the long-standing puzzle. The 1H NMR spectrum and 13C NMR spectrum of the 1-hydroxymethyl-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane were detected by tracking the hydrolysis of PHX, which provide direct experimental evidences for its existence. Meanwhile, it was found that 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane (MNX) is a stable intermediate in the nitrolysis of PHX to generate HMX, and the presence of small amounts of water in the nitrolysis of PHX not only stimulate the redox reaction between nitric acid and formaldehyde but also promote the reaction by hydrolyzing PHX to corresponding N-hydroxymethyl derivative, which verify that 1-hydroxymethyl-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane is the key active intermediate in the synthesis of HMX.
中文翻译:

合成1,3,5,7-四硝基-1,3,5,7-四氮杂环辛烷的关键活性中间体的研究:1-乙酰氧基甲基-3,5,7-三硝基-1,3的水解和硝化, 5,7-四氮杂环辛烷
1-羟基甲基-3,5,7-三硝基-1,3,5,7-四氮杂环辛烷是否是合成1,3,5,7-四硝基-1,3,5,7的关键活性中间体的问题urotropine或3,7-dinitro-1,3,5,7-四氮杂双环[3.3.1]壬烷(DPT)产生的-四氮杂环辛烷(HMX)仍需照明数十年。本文研究了酯化中间体1-乙酰氧基甲基-3,5,7-三硝基-1,3,5,7-四氮杂环辛烷(PHX)的水解和硝化,以解决长期存在的难题。的1 H NMR谱和13通过跟踪PHX的水解来检测1-羟甲基-3,5,7-三硝基-1,3,5,7-四氮杂环辛烷的13 C NMR光谱,这为其存在提供了直接的实验证据。同时发现1-硝基-3,5,7-三硝基-1,3,5,7-四氮杂环辛烷(MNX)是PHX硝化生成HMX的稳定中间体,并且存在少量的PHX硝化过程中的水不仅刺激硝酸与甲醛之间的氧化还原反应,而且通过将PHX水解成相应的N-羟甲基衍生物来促进反应,这证明1-羟甲基-3,5,7-三硝基-1,3 ,5,7-四氮杂环辛烷是合成HMX的关键活性中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号