Forensic Chemistry ( IF 2.6 ) Pub Date : 2021-02-06 , DOI: 10.1016/j.forc.2020.100304 Victoria L. McGuffin , Ruth Waddell Smith
|
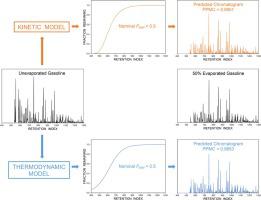
A unified kinetic and thermodynamic model was derived to predict evaporation for forensic applications, such as fire debris analysis. In this model, a reversible first-order reaction served as the foundation, with rate constants (kinetic regime) and standard vapor pressures (thermodynamic regime) as input parameters. The rate constants and standard vapor pressures for normal (n-) alkanes were fit by linear regression to the retention index, with correlation coefficients of 0.9969 and 0.9998, respectively. These regression equations were used to calculate fraction-remaining curves as a function of the retention index. From these curves, the kinetic and thermodynamic models were able to predict the total fraction remaining of the fuel, either as a bulk quantity or as a chromatogram, as well as the fractions remaining of individual compounds.
To evaluate the kinetic and thermodynamic models, gasoline samples were experimentally evaporated to nominal fractions remaining of 0.7, 0.5, 0.3, and 0.1 (30%, 50%, 70% and 90% evaporated). The experimental chromatograms were compared to predicted chromatograms, with Pearson product-moment correlation coefficients of 0.9913 – 0.9068 for the kinetic model and 0.9903 – 0.8921 for the thermodynamic model. The experimental and predicted fractions remaining for individual compounds were compared for n-alkanes and alkylbenzenes spanning a wide range of retention indices and abundances. For the n-alkanes, the mean average percent error was 2.8 – 13.9% for the kinetic model and 3.2 – 20.2% for the thermodynamic model. This approach provides a unified basis for the comparison of the models, and demonstrates the accurate performance of each model.
中文翻译:

用于法医学应用的统一的蒸发动力学和热力学模型
导出了统一的动力学和热力学模型来预测法医应用(例如火屑分析)的蒸发。在此模型中,可逆的一阶反应作为基础,速率常数(动力学状态)和标准蒸气压(热力学状态)作为输入参数。正常(n的速率常数和标准蒸气压。-)烷烃通过线性回归拟合至保留指数,相关系数分别为0.9969和0.9998。这些回归方程用于计算保留分数随保留指数变化的曲线。根据这些曲线,动力学和热力学模型能够以总量或色谱图的形式预测燃料剩余的总馏分以及单个化合物的剩余分馏率。
为了评估动力学模型和热力学模型,将汽油样品实验性地蒸发至标称馏分,剩余量分别为0.7、0.5、0.3和0.1(蒸发了30%,50%,70%和90%)。将实验色谱图与预测色谱图进行比较,动力学模型的皮尔逊积矩相关系数为0.9913 – 0.9068,热力学模型的Pearson积矩相关系数为0.9903 – 0.8921。比较了各个化合物剩余的实验级和预测级分的正构烷烃和烷基苯,它们涵盖了范围广泛的保留指数和丰度。对于n-烷烃,动力学模型的平均平均误差为2.8 – 13.9%,热力学模型的平均平均误差为3.2 – 20.2%。这种方法为模型的比较提供了统一的基础,并演示了每个模型的准确性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号