Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2021-02-07 , DOI: 10.1016/j.saa.2021.119563 Andreas Schwaighofer , Christopher K. Akhgar , Bernhard Lendl
|
Laser-based infrared (IR) spectroscopy is an emerging key technology for the analysis of solutes and for real-time reaction monitoring in liquids. Larger applicable pathlengths compared to the traditional gold standard Fourier transform IR (FTIR) spectroscopy enable robust measurements of analytes in a strongly absorbing matrix such as water. Recent advancements in laser development also provide large accessible spectral coverage thus overcoming an inherent drawback of laser-based IR spectroscopy.
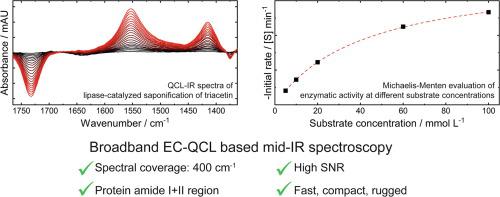
In this work, we benchmark a commercial room temperature operated broadband external cavity-quantum cascade laser (EC-QCL)-IR spectrometer with a spectral coverage of 400 cm−1 against FTIR spectroscopy and showcase its application for measuring the secondary structure of proteins in water, and for monitoring the lipase-catalyzed saponification of triacetin. Regarding the obtained limit of detection (LOD), the laser-based spectrometer compared well to a research-grade FTIR spectrometer employing a liquid nitrogen cooled detector. With respect to a routine FTIR spectrometer equipped with a room temperature operated pyroelectric detector, a 15-fold increase in LOD was obtained in the spectral range of 1600–1700 cm−1. Characteristic spectral features in the amide I and amide II region of three representative proteins with different secondary structures could be measured at concentrations as low as 0.25 mg mL−1. Enzymatic hydrolysis of triacetin by lipase was monitored, demonstrating the advantage of a broad spectral coverage for following complex chemical reactions. The obtained results in combination with the portability and small footprint of the employed spectrometer opens a wide range of future applications in protein analysis and industrial process control, which cannot be readily met by FTIR spectroscopy without recurring to liquid nitrogen cooled detectors.
中文翻译:

基于宽带激光的中红外光谱法,用于蛋白质分析和酶活性监测
基于激光的红外(IR)光谱学是一种新兴的关键技术,用于分析溶质和实时监测液体中的反应。与传统的金标准傅里叶变换红外(FTIR)光谱相比,更大的适用光程使得能够对强吸收性基质(例如水)中的分析物进行可靠的测量。激光开发的最新进展还提供了较大的可访问光谱范围,从而克服了基于激光的红外光谱的固有缺陷。
在这项工作中,我们以FTIR光谱为基准,对光谱范围为400 cm -1的商业室温操作宽带外腔-量子级联激光(EC-QCL)-IR光谱仪进行了标定,并展示了其在测量蛋白质二级结构中的应用。水,并用于监测脂肪酶催化的甘油三乙酸酯皂化。关于获得的检测限(LOD),基于激光的光谱仪与采用液氮冷却检测器的研究级FTIR光谱仪进行了很好的比较。对于配备了室温操作热释电探测器的常规FTIR光谱仪,在1600-1700 cm -1的光谱范围内,LOD增加了15倍。可以在低至0.25 mg mL -1的浓度下测量具有不同二级结构的三种代表性蛋白质在酰胺I和酰胺II区的特征光谱特征。监测了脂肪酶对三醋精的酶促水解,证明了其对于后续复杂化学反应的光谱覆盖范围广。所获得的结果与所用光谱仪的便携性和小占位面积相结合,为蛋白质分析和工业过程控制提供了广阔的未来应用范围,而如果不使用液氮冷却检测器,则FTIR光谱无法轻松满足这些要求。











































 京公网安备 11010802027423号
京公网安备 11010802027423号