Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2021-02-05 , DOI: 10.1016/j.saa.2021.119551 Urban Novak , Jože Grdadolnik
|
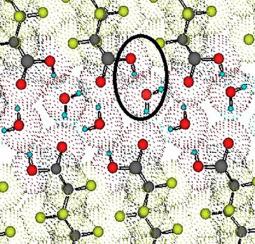
The infrared spectra of the long-chain perfluorocarboxylic acid monohydrates differ markedly from those of the anhydrous dimers. Consequently, the structure of the solid perfluorocarboxylic acid monohydrates must differ from any known dimer-containing carboxylic acid crystals. Consideration of the significant features of the infrared spectra of the long-chain perfluorocarboxylic acid monohydrates, supplemented by their Raman spectra, and comparison with the spectra of auxiliary substances have led us to conclude that the rather strong neutral carboxyl-hydroxyl to water bonding can best explain the observations. The infrared spectra indicate the presence of fairly short hydrogen bonds connecting the water molecules to the carbonyl groups. In the construction of the hydrogen bonding pattern of the perfluorocarboxylic acid monohydrates, the oxalic acid dihydrate plays the key role. The striking similarity between the infrared spectra of the oxalic acid dihydrates and the perfluorocarboxylic acid monohydrates in the regions characteristic of water and O H⋯O vibration suggests that the structure of the hydrated carboxyl groups is the same in both crystals. These regions are characterized by the sharp doublet at 3539 cm−1 and 3464 cm−1, which is due to the H2O ν1 and ν3 stretching vibrations, respectively, and the broad absorption between 3000 cm−1 and 1500 cm−1 with the intense band at 1970 cm−1, both associated with the vibration of the O
H⋯O vibration suggests that the structure of the hydrated carboxyl groups is the same in both crystals. These regions are characterized by the sharp doublet at 3539 cm−1 and 3464 cm−1, which is due to the H2O ν1 and ν3 stretching vibrations, respectively, and the broad absorption between 3000 cm−1 and 1500 cm−1 with the intense band at 1970 cm−1, both associated with the vibration of the O H⋯O group. The later peak consists of two band components at near 1980 cm−1 and 2020 cm−1. These band components show different behaviour when the temperature, polarization or deuteration is changed. In general, the infrared spectra of long-chain perfluorocarboxylic acids represent the system with very short hydrogen bonds connecting the water molecules to the carboxylates. This hydrogen bond pattern should be very similar to that found in the crystals of α-oxalic acid dihydrate.
H⋯O group. The later peak consists of two band components at near 1980 cm−1 and 2020 cm−1. These band components show different behaviour when the temperature, polarization or deuteration is changed. In general, the infrared spectra of long-chain perfluorocarboxylic acids represent the system with very short hydrogen bonds connecting the water molecules to the carboxylates. This hydrogen bond pattern should be very similar to that found in the crystals of α-oxalic acid dihydrate.
中文翻译:

层状全氟羧酸一水合物中氢键网络的红外光谱
长链全氟羧酸一水合物的红外光谱与无水二聚体的红外光谱明显不同。因此,固体全氟羧酸一水合物的结构必须不同于任何已知的含二聚物的羧酸晶体。考虑到长链全氟羧酸一水合物的红外光谱的显着特征,并辅以其拉曼光谱,并与辅助物质的光谱进行比较,我们得出结论,相当强的中性羧基-羟基与水的键合效果最佳解释观察结果。红外光谱表明存在相当短的氢键,将水分子连接到羰基上。在构建全氟羧酸一水合物的氢键模式时,二水草酸起关键作用。草酸二水合物和全氟羧酸一水合物的红外光谱在水和O的特征区域具有惊人的相似性 H = O振动表明两个晶体中的水合羧基结构相同。在3539厘米这些区域的特征是急剧双峰-1和3464厘米-1,这是由于与H 2 öν 1和ν 3的伸缩振动,分别和3000厘米之间的宽吸收-1和1500厘米- 1与1970 cm -1处的强带有关,两者都与O
H = O振动表明两个晶体中的水合羧基结构相同。在3539厘米这些区域的特征是急剧双峰-1和3464厘米-1,这是由于与H 2 öν 1和ν 3的伸缩振动,分别和3000厘米之间的宽吸收-1和1500厘米- 1与1970 cm -1处的强带有关,两者都与O  H⋯O组的振动有关。后面的峰值由1980 cm -1附近和2020 cm -1附近的两个波段组成。当温度,极化或氘改变时,这些能带成分表现出不同的行为。通常,长链全氟羧酸的红外光谱表示具有非常短的氢键的系统,该氢键将水分子连接到羧酸盐上。这种氢键模式应与α-草酸二水合物晶体中的氢键模式非常相似。
H⋯O组的振动有关。后面的峰值由1980 cm -1附近和2020 cm -1附近的两个波段组成。当温度,极化或氘改变时,这些能带成分表现出不同的行为。通常,长链全氟羧酸的红外光谱表示具有非常短的氢键的系统,该氢键将水分子连接到羧酸盐上。这种氢键模式应与α-草酸二水合物晶体中的氢键模式非常相似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号