当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of the Secondary Metal in Ordered and Disordered Pt–M Intermetallic Nanoparticles: An Example of Pt3Sn Nanocubes for the Electrocatalytic Methanol Oxidation
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-04 , DOI: 10.1021/acscatal.0c05370 Hsiang-Sheng Chen 1 , Tania M. Benedetti 1 , Jiaxin Lian 1 , Soshan Cheong 2 , Peter B. O’Mara 1 , Kazeem O. Sulaiman 2 , Cameron H. W. Kelly 1 , Robert W. J. Scott 3 , J. Justin Gooding 1, 4 , Richard D. Tilley 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-04 , DOI: 10.1021/acscatal.0c05370 Hsiang-Sheng Chen 1 , Tania M. Benedetti 1 , Jiaxin Lian 1 , Soshan Cheong 2 , Peter B. O’Mara 1 , Kazeem O. Sulaiman 2 , Cameron H. W. Kelly 1 , Robert W. J. Scott 3 , J. Justin Gooding 1, 4 , Richard D. Tilley 1, 2
Affiliation
|
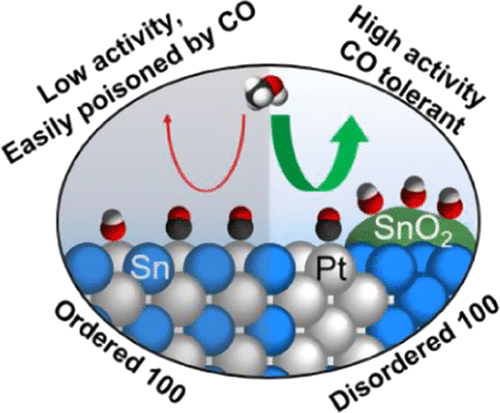
When comparing alloy catalysts with different degrees of ordering, it is important to maintain surface facets to understand the effect of different arrangements of surface atoms. This is even more important when both metals are involved in the reaction steps, which is the case of Pt3Sn for the methanol oxidation reaction (MOR). We have prepared 95 and 60% ordered Pt3Sn nanocubes with {100} facets for the MOR. We show that the Sn atoms in the 60% ordered Pt3Sn nanocubes can be electrochemically oxidized to Sn4+, whereas the Sn atoms in the 95% ordered Pt3Sn nanocubes are more resistant to oxidation. The Sn4+ in the disordered catalysts makes them more active than the ordered catalysts. At low overpotentials, the electrochemically formed Sn4+ in the 60% ordered Pt3Sn nanocubes bind OH, oxidizing the CO intermediate adsorbed on Pt more efficiently. At high overpotentials, Sn4+ prevents the passivation of the Pt sites due to adsorption of OH. These effects lead to a 5.6 times higher activity of the 60% ordered nanocubes compared to the 95% ordered nanocubes. These results illustrate the importance in catalyst design of controlling the environment and especially the atoms neighboring Pt for intermetallic Pt–M electrocatalysts.
中文翻译:

次级金属在有序和无序的Pt–M金属间纳米粒子中的作用:Pt 3 Sn纳米立方体用于电催化甲醇氧化的实例
当比较具有不同有序度的合金催化剂时,重要的是要保持表面刻面,以了解表面原子不同排列的影响。当两种金属都参与反应步骤时,这一点尤为重要,这是甲醇氧化反应(MOR)的Pt 3 Sn的情况。我们已经准备了95%和60%订购的具有{100}刻面的Pt 3 Sn纳米立方体用于MOR。我们显示60%有序的Pt 3 Sn纳米立方体中的Sn原子可以被电化学氧化为Sn 4+,而95%有序的Pt 3 Sn纳米立方体中的Sn原子更耐氧化。锡4+无序催化剂中的α-烯烃使它们比有序催化剂更具活性。在低电势下,60%有序Pt 3 Sn纳米立方体中电化学形成的Sn 4+与OH结合,从而更有效地氧化吸附在Pt上的CO中间体。在高电势下,Sn 4+可防止由于OH的吸附而使Pt部位钝化。这些效应导致60%有序纳米立方体的活性是95%有序纳米立方体的5.6倍。这些结果说明了在催化剂设计中控制环境的重要性,尤其是对于金属间Pt-M电催化剂而言,邻近Pt的原子非常重要。
更新日期:2021-02-19
中文翻译:

次级金属在有序和无序的Pt–M金属间纳米粒子中的作用:Pt 3 Sn纳米立方体用于电催化甲醇氧化的实例
当比较具有不同有序度的合金催化剂时,重要的是要保持表面刻面,以了解表面原子不同排列的影响。当两种金属都参与反应步骤时,这一点尤为重要,这是甲醇氧化反应(MOR)的Pt 3 Sn的情况。我们已经准备了95%和60%订购的具有{100}刻面的Pt 3 Sn纳米立方体用于MOR。我们显示60%有序的Pt 3 Sn纳米立方体中的Sn原子可以被电化学氧化为Sn 4+,而95%有序的Pt 3 Sn纳米立方体中的Sn原子更耐氧化。锡4+无序催化剂中的α-烯烃使它们比有序催化剂更具活性。在低电势下,60%有序Pt 3 Sn纳米立方体中电化学形成的Sn 4+与OH结合,从而更有效地氧化吸附在Pt上的CO中间体。在高电势下,Sn 4+可防止由于OH的吸附而使Pt部位钝化。这些效应导致60%有序纳米立方体的活性是95%有序纳米立方体的5.6倍。这些结果说明了在催化剂设计中控制环境的重要性,尤其是对于金属间Pt-M电催化剂而言,邻近Pt的原子非常重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号