当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The synthesis of 3-(het)aryl-6,7-dihydro-5 H -[1,2,4]-triazolo[3,4- a ][2]benzazepines
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-02-04 , DOI: 10.1007/s10593-021-02868-9 Vladimir А. Glushkov , Dmitry N. Babentsev , Maksim V. Dmitriev , Kseniya А. Stepanova , Anastasiya Yu. Kharintseva , Anastasiya E. Simakhina
中文翻译:

3-(杂)芳基-6,7-二氢-5 H-[1,2,4]-三唑并[3,4- a] [2]苯并ze庚因的合成
更新日期:2021-02-04
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-02-04 , DOI: 10.1007/s10593-021-02868-9 Vladimir А. Glushkov , Dmitry N. Babentsev , Maksim V. Dmitriev , Kseniya А. Stepanova , Anastasiya Yu. Kharintseva , Anastasiya E. Simakhina
|
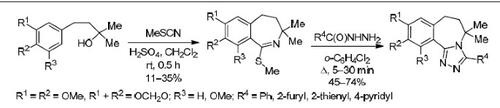
4-Aryl-2-methylbutan-2-ols with methoxy or methylenedioxy substituents participate in the Graf–Ritter reaction with methyl thiocyanate in the presence of an acid (H2SO4, MeSO3H) forming 1-methylsulfanyl-2-benzazepines in low yields (11–35%), which undergo cyclization with benzhydrazide or hetarenecarboxylic acid hydrazides upon reflux in o-dichlorobenzene into the corresponding 3-phenyl- and 3-hetaryl-6,7-dihydro-5H-1,2,4-triazolo[3,4-a][2]benzazepines (45–74% yields).
中文翻译:

3-(杂)芳基-6,7-二氢-5 H-[1,2,4]-三唑并[3,4- a] [2]苯并ze庚因的合成
在存在酸(H 2 SO 4,MeSO 3 H)的情况下,带有甲氧基或亚甲二氧基取代基的4-芳基-2-甲基丁烷-2-醇与硫氰酸甲酯一起参与Graf-Ritter反应,形成1-甲基硫烷基-2-苯并ze庚因收率低(11–35%),在邻二氯苯中回流生成相应的3-苯基-和3-杂芳基-6,7-二氢-5 H -1,2时,可与苄肼或戊烯酰肼进行环化反应, 4-三唑并[3,4- a ] [2]苯并ze庚因(45–74%的产率)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号