当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Trace Tl Removal with Ferrate through the Addition of Mn(II): Effect and Mechanism
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-01-07 , DOI: 10.1021/acsestengg.0c00229 Yu-Lei Liu 1 , Yan-Ting Li 1 , Lu Wang 1 , Wei Wang 1 , Jun Ma 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2021-01-07 , DOI: 10.1021/acsestengg.0c00229 Yu-Lei Liu 1 , Yan-Ting Li 1 , Lu Wang 1 , Wei Wang 1 , Jun Ma 1
Affiliation
|
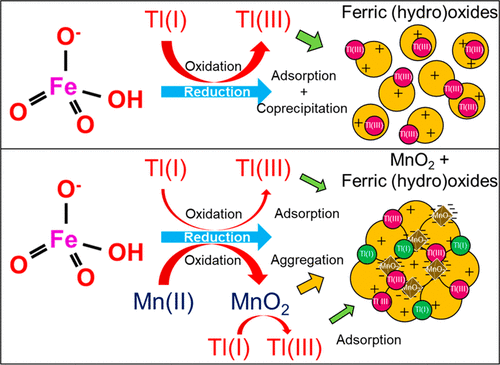
Thallium (Tl) is highly toxic and mobile compared with the other priority pollutant metals, and trace Tl pollution is difficult to control. Some mineral deposits in South China contain high levels of Tl, and Tl related pollution incidents have increasingly occurred in recent years. We previously found that ferrate [K2FeO4, Fe(VI)] could oxidize Tl(I) into Tl(III) and remove the resulting Tl(III). However, the Tl-bearing ferric nanoparticles may penetrate the filtration tank and impact water quality. Herein, with the addition of a low dosage of Mn(II) (50 μg/L), the Tl removal efficiency surged from 42% to 84% [Fe(VI): 0.5 mg/L, Tl: 1 μg/L, pH 7.0]. Time for the formation of 4 μm of floc shortened from 75 min in the Fe(VI) group to 30 min in the Fe(VI) + Mn(II) group. In the reaction process, Mn(II) was oxidized into MnO2, part of Tl(I) was oxidized into Tl(OH)3, and Fe(VI) was reduced into ferric (hydr)oxides. Negatively charged MnO2 (zeta potential: −40.6 mV at pH 7.0) interacted with positively charged ferric (hydr)oxides (zeta potential: 0.8 mV at pH 7.0) through electrostatic attraction with the formation of ferric (hydr)oxides/MnO2 floc. Tl(I) and Tl(OH)3 were adsorbed by the negatively charged ferric (hydr)oxides/MnO2 floc and subsequently removed through solid–liquid separation. By adjusting the molar ratio of Fe(VI)/Mn(II), effective removal of trace Tl was achieved in authentic polluted waters.
更新日期:2021-03-12











































 京公网安备 11010802027423号
京公网安备 11010802027423号