当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular Hydrogen Peroxide Electrosynthesis Cell with Anthraquinone-Modified Polyaniline Electrocatalyst
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2020-12-10 , DOI: 10.1021/acsestengg.0c00173 Qianhong Zhu 1 , Marlena Hinkle 1 , David J. Kim 1 , Jae-Hong Kim 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2020-12-10 , DOI: 10.1021/acsestengg.0c00173 Qianhong Zhu 1 , Marlena Hinkle 1 , David J. Kim 1 , Jae-Hong Kim 1
Affiliation
|
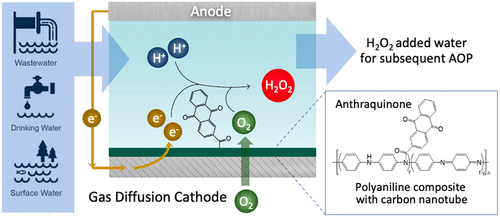
Advanced oxidation processes (AOPs) target the chemical destruction of a wide range of nonbiodegradable, toxic, and recalcitrant organic pollutants instead of removal via physical separation, which produces contaminant-laden concentrates or solids. Hydrogen peroxide (H2O2) is the most widely used precursor that produces a highly reactive and nonselective hydroxyl radical at the site of an AOP through activation by UV irradiation. The potential for AOPs to meet the growing demand of transforming a centralized treatment and distribution practice into a modular, small-scale, and decentralized treatment paradigm can be maximized by innovative technologies that can synthesize precursor chemicals also at the site of water treatment, eliminating the need for a continuous chemical supply. We here present an electrochemical H2O2 generation cell that produces a large quantity of H2O2 while consuming only 0.2 to 20% of the total electricity consumption of AOPs in various AOP application scenarios employing UV activation. We achieve high electrochemical H2O2 production efficiency by synthesizing an anthraquinone-modified polyaniline composite that enables an efficient two-electron oxygen reduction reaction. Polyaniline functions as a conductive support with abundant attachment sites, and anthraquinone ensures selective H2O2 generation. In a flow cell equipped with a gas diffusion cathode, H2O2 can be produced at a rate of 1.80 mol gcatalyst–1 hr–1 at 100 mA with a Faradaic efficiency of 95.83%. Finally, we examined the H2O2 production capability of the device with simulated drinking water and wastewater as feed electrolytes to demonstrate its potential for real-world operation scenarios.
中文翻译:

蒽醌改性聚苯胺电催化剂的模块化过氧化氢电合成池
先进的氧化工艺(AOP)的目标是化学销毁各种不可生物降解,有毒和难降解的有机污染物,而不是通过物理分离进行去除,这会产生富含污染物的浓缩物或固体。过氧化氢(H 2 O 2)是最广泛使用的前体,可通过UV辐射活化在AOP的位点产生高反应性和非选择性的羟基自由基。AOP可以满足日益增长的将集中式处理和分配实践转变为模块化,小规模和分散式处理范式的需求,这可以通过创新技术最大程度地实现,该技术还可以在水处理现场合成前体化学品,从而消除了需要持续的化学品供应。我们在这里提出了一种电化学H 2 O 2产生电池,该电池会产生大量H 2 O 2而在采用紫外线活化的各种AOP应用场景中,其消耗的AOP仅占总耗电量的0.2%到20%。我们通过合成蒽醌改性的聚苯胺复合材料实现了高电化学H 2 O 2的生产效率,该复合物能够实现高效的两电子氧还原反应。聚苯胺用作具有丰富附着位点的导电载体,蒽醌可确保选择性生成H 2 O 2。在配备有气体扩散阴极的流通池中,H 2 O 2的产生速率为1.80 mol g催化剂–1 hr –1在100 mA时的法拉第效率为95.83%。最后,我们使用模拟饮用水和废水作为进料电解质检查了该设备的H 2 O 2生产能力,以证明其在实际操作场景中的潜力。
更新日期:2020-12-10
中文翻译:

蒽醌改性聚苯胺电催化剂的模块化过氧化氢电合成池
先进的氧化工艺(AOP)的目标是化学销毁各种不可生物降解,有毒和难降解的有机污染物,而不是通过物理分离进行去除,这会产生富含污染物的浓缩物或固体。过氧化氢(H 2 O 2)是最广泛使用的前体,可通过UV辐射活化在AOP的位点产生高反应性和非选择性的羟基自由基。AOP可以满足日益增长的将集中式处理和分配实践转变为模块化,小规模和分散式处理范式的需求,这可以通过创新技术最大程度地实现,该技术还可以在水处理现场合成前体化学品,从而消除了需要持续的化学品供应。我们在这里提出了一种电化学H 2 O 2产生电池,该电池会产生大量H 2 O 2而在采用紫外线活化的各种AOP应用场景中,其消耗的AOP仅占总耗电量的0.2%到20%。我们通过合成蒽醌改性的聚苯胺复合材料实现了高电化学H 2 O 2的生产效率,该复合物能够实现高效的两电子氧还原反应。聚苯胺用作具有丰富附着位点的导电载体,蒽醌可确保选择性生成H 2 O 2。在配备有气体扩散阴极的流通池中,H 2 O 2的产生速率为1.80 mol g催化剂–1 hr –1在100 mA时的法拉第效率为95.83%。最后,我们使用模拟饮用水和废水作为进料电解质检查了该设备的H 2 O 2生产能力,以证明其在实际操作场景中的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号