当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NO2 Removal by Adsorption on Transition-Metal-Based Layered Double Hydroxides
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2020-12-04 , DOI: 10.1021/acsestengg.0c00121 Shanshan Shang 1, 2 , Chao Yang 3 , Yuanmeng Tian 1, 2 , Zeyu Tao 1, 2 , Aamir Hanif 1, 2, 4 , Mingzhe Sun 1, 2 , Ho Hin Stephen Wong 1 , Chenguang Wang 5 , Jin Shang 1, 2
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2020-12-04 , DOI: 10.1021/acsestengg.0c00121 Shanshan Shang 1, 2 , Chao Yang 3 , Yuanmeng Tian 1, 2 , Zeyu Tao 1, 2 , Aamir Hanif 1, 2, 4 , Mingzhe Sun 1, 2 , Ho Hin Stephen Wong 1 , Chenguang Wang 5 , Jin Shang 1, 2
Affiliation
|
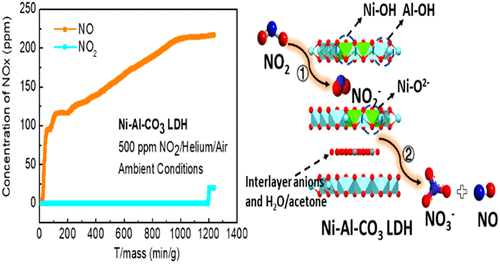
The emission of nitrogen dioxide (NO2) has caused severe air pollution and threatened the safety of the environment and people’s health. Various techniques have been intensively explored for the abatement of NO2, mostly on the basis of catalytic reduction at elevated temperature, but few have shown satisfactory NO2 removal efficiency at ambient conditions. The use of solid porous adsorbents is a promising approach for NO2 removal due to their high capacity and low energy penalty for regeneration. Here, we report the uncalcined transition-metal-based layered double hydroxides (TM–Al–CO3 LDHs) as ambient NO2 adsorbents. The dynamic breakthrough experiments demonstrated that Ni–Al–CO3 LDH showed a superior NO2 adsorption capacity above 5.3 mmol g–1 and the lowest NO generation ratio (∼31.7% of the total NO2 input) among the four TM–Al–CO3 adsorbents. The in situ diffuse reflectance infrared Fourier transform spectroscopy disclosed the reactive adsorption mechanism between NO2 and LDHs via acid–base interaction. The reversibility of active adsorption sites in Ni–Al–CO3 LDH could maintain over 74% after six adsorption–desorption cycles, suggesting a decent regenerability of Ni–Al–CO3 as the NO2 adsorbent.
中文翻译:

过渡金属基层状双金属氢氧化物吸附去除NO 2
二氧化氮(NO 2)的排放已造成严重的空气污染,并威胁到环境安全和人民健康。为了减少NO 2,已经进行了广泛的探索,主要是基于在高温下的催化还原,但是很少有人在环境条件下表现出令人满意的NO 2去除效率。固体多孔吸附剂的使用由于其高容量和较低的再生能耗而成为一种有希望的NO 2去除方法。在这里,我们报告了未煅烧的过渡金属基层状双氢氧化物(TM-Al-CO 3 LDHs)作为环境NO 2吸附剂。动态突破实验表明,Ni-Al-CO3 LDH在四种TM–Al–CO 3吸附剂中均显示出在5.3 mmol g –1以上的情况下具有出色的NO 2吸附能力,并且最低的NO产生率(约占总NO 2输入量的31.7%)。该原位漫反射傅里叶变换红外光谱中公开NO之间的反应性吸附机构2经由酸-碱相互作用和水滑石。在六个吸附-解吸循环后,Ni-Al-CO 3 LDH中活性吸附位点的可逆性可以保持74%以上,这表明Ni-Al-CO 3作为NO 2吸附剂具有良好的可再生性。
更新日期:2020-12-04
中文翻译:

过渡金属基层状双金属氢氧化物吸附去除NO 2
二氧化氮(NO 2)的排放已造成严重的空气污染,并威胁到环境安全和人民健康。为了减少NO 2,已经进行了广泛的探索,主要是基于在高温下的催化还原,但是很少有人在环境条件下表现出令人满意的NO 2去除效率。固体多孔吸附剂的使用由于其高容量和较低的再生能耗而成为一种有希望的NO 2去除方法。在这里,我们报告了未煅烧的过渡金属基层状双氢氧化物(TM-Al-CO 3 LDHs)作为环境NO 2吸附剂。动态突破实验表明,Ni-Al-CO3 LDH在四种TM–Al–CO 3吸附剂中均显示出在5.3 mmol g –1以上的情况下具有出色的NO 2吸附能力,并且最低的NO产生率(约占总NO 2输入量的31.7%)。该原位漫反射傅里叶变换红外光谱中公开NO之间的反应性吸附机构2经由酸-碱相互作用和水滑石。在六个吸附-解吸循环后,Ni-Al-CO 3 LDH中活性吸附位点的可逆性可以保持74%以上,这表明Ni-Al-CO 3作为NO 2吸附剂具有良好的可再生性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号