Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-02-03 , DOI: 10.1016/j.atmosenv.2021.118242 Ze-Gang Dong , Fang Xu , Ellen Mitchell , Bo Long
|
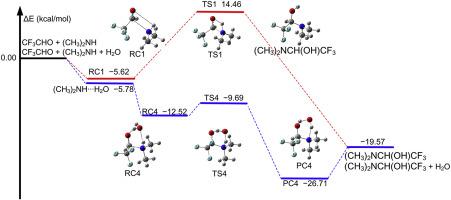
High-level ab initio calculations and variational transition state theory with small curvature tunneling have been used to study the aminolysis of trifluoroacetaldehyde catalyzed by a single water molecule. The results of energetic studies indicate that the energy barrier of the trifluoroacetaldehyde aminolysis reaction decreases along ammonia, methylamine, and dimethylamine. A single water molecule can significantly reduce the reaction energy barrier of trifluoroacetaldehyde aminolysis. In particular, the reaction involving dimethylamine has the lowest reaction energy barrier and the energy barrier is decreased to be −9.69 kcal/mol in the CF3CHO + (CH3)2NH + H2O reaction relative to CF3CHO, (CH3)2NH, and H2O separated reactants. Kinetic calculation shows that the rate coefficient of CF3CHO + (CH3)2NH⋯H2O ranges from 7.78 × 10−13 to 4.45 × 10−16 cm3⋅molecules−1⋅s−1 at 190–350 K. Here, we find an important CF3CHO elimination pathway, which can compete with the reaction of CF3CHO + OH when the OH concentration is 104 molecules⋅cm−3 and the dimethylamine concentration is higher than 109 molecules⋅cm−3 in the temperature range between 240 and 330 K. In addition, once (CH3)2NCH(OH)CF3 is formed by the reaction of CF3CHO + (CH3)2NH + H2O, it will further promote the growth of secondary organic aerosols.
中文翻译:

一个水分子催化的三氟乙醛氨解:一个重要的三氟乙醛沉通道和一个潜在的二次有机气溶胶生长途径
高水平的从头计算和具有小曲率隧穿的变迁过渡态理论已被用于研究单个水分子催化的三氟乙醛的氨解反应。高能研究的结果表明,三氟乙醛氨解反应的能垒沿氨,甲胺和二甲胺而降低。单个水分子可以显着降低三氟乙醛氨解反应的反应能垒。特别地,涉及二甲胺的反应具有最低的反应能垒,并且相对于CF 3 CHO,在CF 3 CHO +(CH 3)2 NH + H 2 O反应中,能垒降低为-9.69 kcal / mol。CH3)2 NH和H 2 O分离的反应物。动力学计算表明,CF的速率系数3 CHO +(CH 3)2 NH⋯ħ 2 O范围从7.78×10 -13到4.45×10 -16 厘米3 ⋅molecules -1 ⋅s -1在190-350ķ在这里,我们发现了一条重要的CF 3 CHO消除途径,当OH浓度为10 4分子· cm -3且二甲胺浓度高于10 9分子· cm时,可以与CF 3 CHO + OH的反应竞争。-3在240至330 K的温度范围内。另外,一旦通过CF 3 CHO +(CH 3)2 NH + H 2 O的反应形成(CH 3)2 NCH(OH)CF 3,它将进一步促进二次有机气溶胶的生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号