当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Behavior and Cost-Effectiveness of Halogen Radicals in Hg0 Removal: Performance, Kinetics, and Mechanism
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2020-10-01 , DOI: 10.1021/acsestengg.0c00020 Runlong Hao 1, 2 , Zheng Wang 1 , Chen Tang 1 , Zhengyang Gao 3 , Bo Yuan 1, 2 , Weijie Yang 3 , Yi Zhao 1, 2
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2020-10-01 , DOI: 10.1021/acsestengg.0c00020 Runlong Hao 1, 2 , Zheng Wang 1 , Chen Tang 1 , Zhengyang Gao 3 , Bo Yuan 1, 2 , Weijie Yang 3 , Yi Zhao 1, 2
Affiliation
|
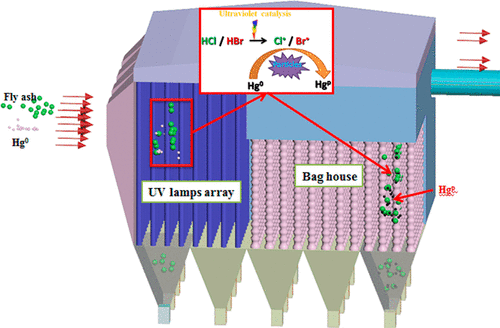
We used ultraviolet and heat (UV-heat) to costimulate HCl, HBr, and HI to yield Cl•, Br•, and I• for Hg0 removal. Electron spin resonance (ESR) confirmed the radical formation. Cl•/Br•/I• were found to play the leading roles in Hg0 removal, Br2/I2 formed from the heat catalysis of HBr/HI also played key roles. The costs for removing 80% Hg0 with UV-heat/HCl, UV-heat/HBr, and UV-heat/HI were calculated to be 0.03, 2.4, and 36.9 USD/lb-Hg0, respectively. Rising heat catalysis temperature favored the Hg0 removal. Declining flue gas residence time and adding NO and SO2 impaired the Hg0 removal by Cl• and Br• but did not include I•. Kinetics analyses indicated that UV increased the Hg0 removal rate by 15.5 times for HCl, by 1.3 times for HBr, and by 0.2 time for HI. Radical quenching test with tertiary butanol (TBA) and CO2 indicated that Cl• and Cl2•– contributed almost 100% and <5% to Hg0 removal, respectively. Density functional theory (DFT) calculation gives the reactivity order of oxidizing species toward Hg0, that is, Cl• > Br• > I• > I2 > Br2 > Cl2. The environmetnal implications were proposed finally.
中文翻译:

Hg 0去除中卤素自由基的反应行为和成本-效果:性能,动力学和机理
我们使用紫外线和热能(UV-heat)共同估算HCl,HBr和HI,以生成Cl •,Br •和I •来去除Hg 0。电子自旋共振(ESR)证实了自由基的形成。发现Cl • / Br • / I •在Hg 0去除中起主要作用,由HBr / HI的热催化形成的Br 2 / I 2也起关键作用。计算出用紫外线加热/ HCl,紫外线加热/ HBr和紫外线加热/ HI去除80%Hg 0的成本分别为0.03、2.4和36.9 USD / lb-Hg 0。热催化温度升高有利于汞0去除。降低烟气停留时间并添加NO和SO 2会损害Cl •和Br •去除Hg 0的能力,但不包括I •。动力学分析表明,对于HCl,UV将Hg 0的去除率提高了15.5倍,对于HBr,提高了1.3倍,对于HI,提高了0.2倍。用叔丁醇(TBA)和CO 2进行的自由基淬灭试验表明,Cl •和Cl 2 • –分别对Hg 0的去除贡献了近100%和<5%。密度泛函理论(DFT)计算给出了氧化物质对Hg 0(Cl )的反应顺序• > Br • > I • > I 2 > Br 2 > Cl 2。最后提出了环境意义。
更新日期:2020-10-01
中文翻译:

Hg 0去除中卤素自由基的反应行为和成本-效果:性能,动力学和机理
我们使用紫外线和热能(UV-heat)共同估算HCl,HBr和HI,以生成Cl •,Br •和I •来去除Hg 0。电子自旋共振(ESR)证实了自由基的形成。发现Cl • / Br • / I •在Hg 0去除中起主要作用,由HBr / HI的热催化形成的Br 2 / I 2也起关键作用。计算出用紫外线加热/ HCl,紫外线加热/ HBr和紫外线加热/ HI去除80%Hg 0的成本分别为0.03、2.4和36.9 USD / lb-Hg 0。热催化温度升高有利于汞0去除。降低烟气停留时间并添加NO和SO 2会损害Cl •和Br •去除Hg 0的能力,但不包括I •。动力学分析表明,对于HCl,UV将Hg 0的去除率提高了15.5倍,对于HBr,提高了1.3倍,对于HI,提高了0.2倍。用叔丁醇(TBA)和CO 2进行的自由基淬灭试验表明,Cl •和Cl 2 • –分别对Hg 0的去除贡献了近100%和<5%。密度泛函理论(DFT)计算给出了氧化物质对Hg 0(Cl )的反应顺序• > Br • > I • > I 2 > Br 2 > Cl 2。最后提出了环境意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号