当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Steam Reforming of Ethanol Using Ni–Co Catalysts Supported on MgAl2O4: Structural Study and Catalytic Properties at Different Temperatures
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acscatal.0c03351 Adriano H. Braga 1 , Daniela C. de Oliveira 2 , Alan R. Taschin 1 , João B. O. Santos 1 , Jean Marcel R. Gallo 3 , José M. C. Bueno 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acscatal.0c03351 Adriano H. Braga 1 , Daniela C. de Oliveira 2 , Alan R. Taschin 1 , João B. O. Santos 1 , Jean Marcel R. Gallo 3 , José M. C. Bueno 1
Affiliation
|
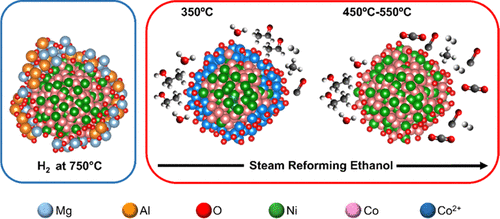
A spectroscopic and microscopic investigation was made of the dynamics of bimetallic nanoparticles (NPs) in Co–Ni/MgAl2O4 catalysts used for the steam reforming of ethanol (SRE) reaction, considering the implications for catalytic performance, shedding light on the elusive effect of Co–Ni alloy in reforming reactions. X-ray absorption spectroscopy (XAS) analyses showed that contact with the reagent mixture led to major changes in the superficial structure of the Co–Ni nanoparticles, which were strongly dependent on temperature and the metal particle size. At room temperature, contact between the Co–Ni NPs and the reactants (ethanol and H2O) led to the formation of a Co–Ni oxide film over the Co–Ni metal core, with CoO/NiO = 1. For smaller Co–Ni NPs (about 5 nm), the oxide film showed a dynamic composition according to temperature, with Co migrating to the surface while being oxidized up to around 350 °C. A structure with a core of (Co,Ni) and a shell rich in CoO was formed, which could be reduced above 350 °C. The surface CoO was mainly reduced upon heating in the reaction stream, with migration into the NP cores. For larger Co–Ni NPs (about 10 nm), the oxide film remained stable up to 200 °C, being reduced by the ethanol stream at higher temperatures. At operating temperatures in the range of 350–400 °C, the surface structure of the Ni–Co alloy showed (Ni,Co)–O species, while these metal oxide species decreased with increases of the thermal treatment temperature and the nanoparticle size. At low temperatures, the Co–Ni nanoparticles were covered by CoO/NiO and were active for the oxidative dehydrogenation of ethanol. With the increase of temperature, surface CoO was reduced and incorporated in the Co–Ni nanoparticle core. These metal sites became active toward the reactant and catalyzed the dehydrogenation of ethanol, followed by C–C bond cleavage, resulting in the formation of CH4, CO, and H2. The presence of CoO at the surface of the smaller Co–Ni nanoparticles suppressed carbon accumulation, compared to larger Co–Ni or Ni nanoparticles that had highly reduced surfaces.
中文翻译:

MgAl 2 O 4负载Ni-Co催化剂对乙醇进行水蒸气重整:不同温度下的结构研究和催化性能
对用于乙醇蒸汽重整(SRE)反应的Co-Ni / MgAl 2 O 4催化剂中双金属纳米颗粒(NPs)的动力学进行了光谱和显微镜研究,考虑到对催化性能的影响,使难以捉摸的光明镍钴合金在重整反应中的作用。X射线吸收光谱法(XAS)分析表明,与试剂混合物的接触导致Co-Ni纳米颗粒的表面结构发生重大变化,这主要取决于温度和金属粒径。在室温下,Co-Ni NP与反应物(乙醇和H 2O)导致在Co-Ni金属芯上形成Co-Ni氧化膜,CoO / NiO =1。对于较小的Co-Ni NP(约5 nm),该氧化膜根据温度显示出动态成分,其中Co在氧化到约350°C时迁移到表面。形成具有(Co,Ni)核和富含CoO的壳的结构,可以将其降低到350°C以上。在反应流中加热时,表面CoO主要被还原,并迁移到NP核中。对于较大的Co-Ni NPs(约10 nm),氧化膜在高达200°C的温度下仍保持稳定,并在较高温度下被乙醇流所还原。在350-400°C的工作温度范围内,Ni-Co合金的表面结构显示(Ni,Co)-O物种,这些金属氧化物随着热处理温度和纳米颗粒尺寸的增加而减少。在低温下,Co-Ni纳米颗粒被CoO / NiO覆盖,并且对乙醇的氧化脱氢具有活性。随着温度的升高,表面CoO减少并被掺入Co-Ni纳米颗粒核中。这些金属位点对反应物具有活性,并催化乙醇的脱氢反应,然后进行CC键断裂,从而形成CH4,CO和H 2。与表面高度还原的较大的Co-Ni或Ni纳米颗粒相比,较小的Co-Ni纳米颗粒表面存在CoO抑制了碳积累。
更新日期:2021-02-19
中文翻译:

MgAl 2 O 4负载Ni-Co催化剂对乙醇进行水蒸气重整:不同温度下的结构研究和催化性能
对用于乙醇蒸汽重整(SRE)反应的Co-Ni / MgAl 2 O 4催化剂中双金属纳米颗粒(NPs)的动力学进行了光谱和显微镜研究,考虑到对催化性能的影响,使难以捉摸的光明镍钴合金在重整反应中的作用。X射线吸收光谱法(XAS)分析表明,与试剂混合物的接触导致Co-Ni纳米颗粒的表面结构发生重大变化,这主要取决于温度和金属粒径。在室温下,Co-Ni NP与反应物(乙醇和H 2O)导致在Co-Ni金属芯上形成Co-Ni氧化膜,CoO / NiO =1。对于较小的Co-Ni NP(约5 nm),该氧化膜根据温度显示出动态成分,其中Co在氧化到约350°C时迁移到表面。形成具有(Co,Ni)核和富含CoO的壳的结构,可以将其降低到350°C以上。在反应流中加热时,表面CoO主要被还原,并迁移到NP核中。对于较大的Co-Ni NPs(约10 nm),氧化膜在高达200°C的温度下仍保持稳定,并在较高温度下被乙醇流所还原。在350-400°C的工作温度范围内,Ni-Co合金的表面结构显示(Ni,Co)-O物种,这些金属氧化物随着热处理温度和纳米颗粒尺寸的增加而减少。在低温下,Co-Ni纳米颗粒被CoO / NiO覆盖,并且对乙醇的氧化脱氢具有活性。随着温度的升高,表面CoO减少并被掺入Co-Ni纳米颗粒核中。这些金属位点对反应物具有活性,并催化乙醇的脱氢反应,然后进行CC键断裂,从而形成CH4,CO和H 2。与表面高度还原的较大的Co-Ni或Ni纳米颗粒相比,较小的Co-Ni纳米颗粒表面存在CoO抑制了碳积累。











































 京公网安备 11010802027423号
京公网安备 11010802027423号