当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Boron–Oxygen Transborylation Strategy for a Catalytic Midland Reduction
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acscatal.0c05168 Kieran Nicholson 1 , Joanne Dunne 1 , Peter DaBell 1 , Alexander Beaton Garcia 1 , Andrew D. Bage 1 , Jamie H. Docherty 1 , Thomas A. Hunt 2 , Thomas Langer 3 , Stephen P. Thomas 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acscatal.0c05168 Kieran Nicholson 1 , Joanne Dunne 1 , Peter DaBell 1 , Alexander Beaton Garcia 1 , Andrew D. Bage 1 , Jamie H. Docherty 1 , Thomas A. Hunt 2 , Thomas Langer 3 , Stephen P. Thomas 1
Affiliation
|
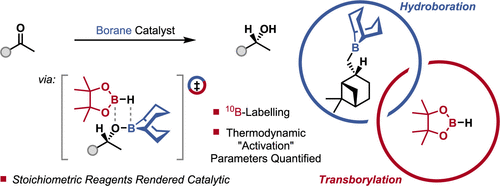
The enantioselective hydroboration of ketones is a textbook reaction requiring stoichiometric amounts of an enantioenriched borane, with the Midland reduction being a seminal example. Here, a turnover strategy for asymmetric catalysis, boron–oxygen transborylation, has been developed and used to transform the stoichiometric borane reagents of the Midland reduction into catalysts. This turnover strategy was demonstrated by the enantioselective reduction of ketones, including derivatives of biologically active molecules and those containing reducible groups. The enantioenriched borane catalyst was generated in situ from commercially available reagents, 9-borabicyclo[3.3.1]nonane (H-B-9-BBN) and β-pinene, and B–O transborylation with pinacolborane (HBpin) was used for catalytic turnover. Mechanistic studies indicated that B–O transborylation proceeded by B–O/B–H boron exchange through a stereoretentive, concerted transition state, resembling σ-bond metathesis.
中文翻译:

硼-氧离子交换策略催化中部还原
酮的对映选择性氢硼化是教科书上的反应,需要化学计量的对映体富集的硼烷,Midland还原是一个开创性的例子。在这里,已经开发出了一种用于不对称催化的转换策略,即硼-氧转硼化反应,并将其用于将Midland还原反应的化学计量硼烷试剂转化为催化剂。这种转换策略通过酮的对映选择性还原得到证实,其中包括生物活性分子的衍生物和含有可还原基团的酮。在生成对映体富集的硼烷催化剂在原位从市售的试剂,9-硼杂双环[3.3.1]壬烷(H-乙-9-BBN)和β-pine烯,以及频哪醇硼烷(HBpin)进行的B–O硼氢化反应用于催化转换。机理研究表明,B–O / B–H硼交换通过立体固位,一致的过渡态(类似于σ键复分解)使B–O发生转硼化作用。
更新日期:2021-02-19
中文翻译:

硼-氧离子交换策略催化中部还原
酮的对映选择性氢硼化是教科书上的反应,需要化学计量的对映体富集的硼烷,Midland还原是一个开创性的例子。在这里,已经开发出了一种用于不对称催化的转换策略,即硼-氧转硼化反应,并将其用于将Midland还原反应的化学计量硼烷试剂转化为催化剂。这种转换策略通过酮的对映选择性还原得到证实,其中包括生物活性分子的衍生物和含有可还原基团的酮。在生成对映体富集的硼烷催化剂在原位从市售的试剂,9-硼杂双环[3.3.1]壬烷(H-乙-9-BBN)和β-pine烯,以及频哪醇硼烷(HBpin)进行的B–O硼氢化反应用于催化转换。机理研究表明,B–O / B–H硼交换通过立体固位,一致的过渡态(类似于σ键复分解)使B–O发生转硼化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号