当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Mechanistic Requirements for Efficient and Stereoselective Alkene Epoxidation by a Cytochrome P450 Enzyme
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acscatal.0c04872 Tom Coleman 1 , Alicia M. Kirk 2 , Rebecca R. Chao 1 , Matthew N. Podgorski 1 , Joshua S. Harbort 3 , Luke R. Churchman 2 , John B. Bruning 4 , Paul V. Bernhardt 2 , Jeffrey R. Harmer 3 , Elizabeth H. Krenske 2 , James J. De Voss 2 , Stephen G. Bell 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-01 , DOI: 10.1021/acscatal.0c04872 Tom Coleman 1 , Alicia M. Kirk 2 , Rebecca R. Chao 1 , Matthew N. Podgorski 1 , Joshua S. Harbort 3 , Luke R. Churchman 2 , John B. Bruning 4 , Paul V. Bernhardt 2 , Jeffrey R. Harmer 3 , Elizabeth H. Krenske 2 , James J. De Voss 2 , Stephen G. Bell 1
Affiliation
|
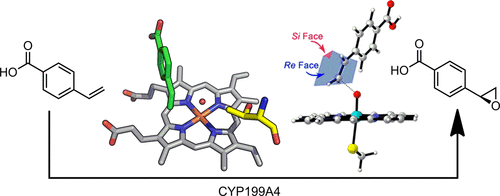
The cytochrome P450 (CYP) family of heme monooxygenase enzymes commonly catalyzes enantioselective hydroxylation and epoxidation reactions. Epoxidation reactions have been hypothesized to proceed via multiple mechanisms involving different reactive intermediates. Here, we use activity, spectroscopic, structural, and molecular dynamics data to investigate the activity and stereoselectivity of 4-vinylbenzoic acid epoxidation by the bacterial enzyme CYP199A4 from Rhodopseudomonas palustris HaA2. The epoxidation of 4-vinylbenzoic acid by CYP199A4 proceeded with high enantioselectivity, giving the (S)-epoxide in 99% ee at an activity of 220 nmol nmol-CYP–1 min–1. Optical and EPR spectroscopy, redox potential measurements, and the crystal structure of 4-vinylbenzoic acid-bound CYP199A4 indicated the partial retention of an aqua ligand at the heme center in the presence of the substrate, providing a justification of the lower activity (∼20%) compared to the oxidative demethylation of 4-methoxybenzoic acid. Mutagenesis at the conserved acid–alcohol pair (D251/T252), which perturbs the generation of the reactive oxygen intermediates, was employed to investigate their role in epoxidation reactions. The T252A mutant increased the rate of turnover of the catalytic cycle, but an elevation in hydrogen peroxide generation via uncoupling resulted in a similar rate of epoxide formation. The activity of epoxidation significantly reduced with the D251N mutant. The chemoselectivity and stereoselectivity of the epoxidation reaction were maintained in the turnovers by these mutants. Overall, there was little evidence that other intermediates, aside from the archetypal reactive ferryl porphyrin cation radical, Compound I, contributed significantly to the epoxidation reaction. The observation of the high selectivity for the (S)-enantiomer was rationalized by molecular dynamics simulations. When the arrangement of the alkene and the active intermediate approached an ideal transition state structure for epoxidation, one face of the alkene was more often exposed to the iron oxo unit.
中文翻译:

了解通过细胞色素P450酶进行高效和立体选择性烯烃环氧化的机理要求
血红素单加氧酶的细胞色素P450(CYP)家族通常催化对映选择性羟基化和环氧化反应。已经假设环氧化反应是通过涉及不同反应性中间体的多种机理进行的。在这里,我们使用活性,光谱,结构和分子动力学数据来研究4-甲基苯甲酸环氧化的活性和立体选择性,CYP199A4是由Phodopseudomonas palustris HaA2产生的。CYP199A4对4-乙烯基苯甲酸的环氧化反应具有很高的对映选择性,在99%ee中得到(S)-环氧化物,活性为220 nmol nmol-CYP –1 min –1。光学和EPR光谱,氧化还原电势测量以及与4-乙烯基苯甲酸结合的CYP199A4的晶体结构表明,在存在底物的情况下,aqua配体在血红素中心部分保留,提供了较低活性的证明(〜20 %)与4-甲氧基苯甲酸的氧化脱甲基相比。保守的酸-醇对(D251 / T252)的诱变会扰乱活性氧中间体的生成,被用来研究其在环氧化反应中的作用。T252A突变体增加了催化循环的周转率,但通过解偶联导致相似的环氧化物形成速率。D251N突变体使环氧化活性大大降低。这些突变体在营业额中保持了环氧化反应的化学选择性和立体选择性。总体而言,几乎没有证据表明,除了原型反应性阿魏卟啉阳离子基团化合物I外,其他中间体也对环氧化反应做出了重要贡献。(S)-对映异构体的高选择性的观察是通过分子动力学模拟合理化的。当烯烃和活性中间体的排列接近用于环氧化的理想过渡态结构时,烯烃的一个面更经常暴露于氧化铁单元。
更新日期:2021-02-19
中文翻译:

了解通过细胞色素P450酶进行高效和立体选择性烯烃环氧化的机理要求
血红素单加氧酶的细胞色素P450(CYP)家族通常催化对映选择性羟基化和环氧化反应。已经假设环氧化反应是通过涉及不同反应性中间体的多种机理进行的。在这里,我们使用活性,光谱,结构和分子动力学数据来研究4-甲基苯甲酸环氧化的活性和立体选择性,CYP199A4是由Phodopseudomonas palustris HaA2产生的。CYP199A4对4-乙烯基苯甲酸的环氧化反应具有很高的对映选择性,在99%ee中得到(S)-环氧化物,活性为220 nmol nmol-CYP –1 min –1。光学和EPR光谱,氧化还原电势测量以及与4-乙烯基苯甲酸结合的CYP199A4的晶体结构表明,在存在底物的情况下,aqua配体在血红素中心部分保留,提供了较低活性的证明(〜20 %)与4-甲氧基苯甲酸的氧化脱甲基相比。保守的酸-醇对(D251 / T252)的诱变会扰乱活性氧中间体的生成,被用来研究其在环氧化反应中的作用。T252A突变体增加了催化循环的周转率,但通过解偶联导致相似的环氧化物形成速率。D251N突变体使环氧化活性大大降低。这些突变体在营业额中保持了环氧化反应的化学选择性和立体选择性。总体而言,几乎没有证据表明,除了原型反应性阿魏卟啉阳离子基团化合物I外,其他中间体也对环氧化反应做出了重要贡献。(S)-对映异构体的高选择性的观察是通过分子动力学模拟合理化的。当烯烃和活性中间体的排列接近用于环氧化的理想过渡态结构时,烯烃的一个面更经常暴露于氧化铁单元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号