Hydrometallurgy ( IF 4.8 ) Pub Date : 2021-01-30 , DOI: 10.1016/j.hydromet.2021.105568 Elena Romanovskaia , Valentin Romanovski , Witold Kwapinski , Irina Kurilo
|
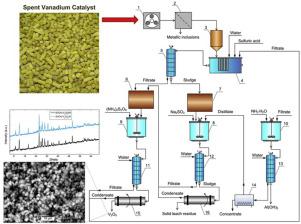
Spent vanadium catalysts of sulfuric acid production (main elemental composition in wt%: 7.5 V, 9.1 K, 10.2 S, 23.2 Si and 1.4 Fe) can be used as a secondary source of vanadium. Extraction of vanadium was studied using two-step leaching (acidic and reductive) of spent vanadium catalysts with further oxidizing of leaching solutions. The factors leaching and hydrolysis temperature, concentration of leaching (H2SO4, Na2SO3) and oxidizing ((NH4)2S2O8) reagents, solid/liquid ratio, mixing parameters, and time of leaching and thermohydrolysis were systematically investigated. The solubility of V2O5 was investigated as a function of temperature, pH of sulfuric acid solutions, and concentration of Na2SO3. The kinetics of V2O5 solubility and reduction were also studied. The vanadium leaching yield after a two-step recovery was 98 wt% after acidic (H2SO4, pH 1.2–1.3) leaching with ultrasonic treatment for 5 min at ambient temperature, followed by reductive leaching in 0.01 Mol/L Na2SO3 solution for 15 min at ambient temperature. The highest vanadium extraction yield from leaching solutions was 98 wt% obtained through oxidizing of leaching solutions by 30 wt%. (NH4)2S2O8 with a molar ratio n(V2O5)/n((NH4)2S2O8) of 5/1 for a reaction time of 5 min at 80–90 °C. the extracted vanadium product was V2O5 with a purity of 85–87 wt%. The technological scheme has been developed to recycle all obtained products and sub-products
中文翻译:

从硫酸生产的废催化剂中选择性回收五氧化二钒:可持续方法
硫酸生产用过的钒催化剂(主要元素组成,以重量%计:7.5 V,9.1 K,10.2 S,23.2 Si和1.4 Fe)可用作钒的辅助来源。研究了钒的萃取过程,该过程使用了两步浸出的钒催化剂(酸性和还原性),并进一步氧化了浸出液。浸出和水解温度,浸出浓度(H 2 SO 4,Na 2 SO 3)和氧化性((NH 4)2 S 2 O 8)试剂,固/液比,混合参数以及浸出和热水解时间的因素被系统地调查。V 2 O 5的溶解度根据温度,硫酸溶液的pH值和Na 2 SO 3的浓度来研究碳氢化合物。还研究了V 2 O 5溶解和还原的动力学。酸性(H 2 SO 4,pH 1.2-1.3)在环境温度下超声浸提5分钟后,再进行0.01 Mol / L Na 2 SO的还原浸提,经过两步回收后,钒的浸提产率为98 wt%。3溶液在环境温度下放置15分钟。通过将浸出溶液氧化30重量%,从浸出溶液中最高的钒提取产率为98重量%。(NH 4)2 S2 O 8的摩尔比n(V 2 O 5)/ n((NH 4)2 S 2 O 8)为5/1,在80–90°C下反应时间为5分钟。提取的钒产品为V 2 O 5,纯度为85-87 wt%。已经开发了技术方案以回收所有获得的产品和副产品











































 京公网安备 11010802027423号
京公网安备 11010802027423号