Applied Surface Science ( IF 6.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2021.149179 Jian-Rong Li , Wan-Peng Zhang , Chang Li , Hang Xiao , Chi He
|
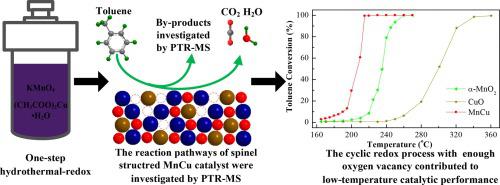
Developing facile preparation method to obtain the satisfied low-temperature catalytic performance of transitional metal oxide-based materials is still a challenge in deep degradation of VOCs. Here, a series of Mn-Cu bimetallic oxide catalysts were prepared by one-step hydrothermal-redox method for catalytic total oxidation of toluene. The CH3COOH concentration, Cu/Mn molar ratio and calcination temperature greatly affected the crystal structure, micromorphology and catalytic performance. Amongst, MnCu spinel structured catalyst exhibited excellent low-temperature catalytic activity, superior durability and water resistance in toluene total oxidation owing to its abundant surface adsorbed oxygen species, higher amount of Cu+ and Mn3+ and excellent low-temperature reducibility. The reaction rate of MnCu was 7.0 times higher than that of MnCu0.5 at 210 °C. The cyclic redox process with enough oxygen vacancy played a vital role in toluene oxidation. The deep oxidation of benzene was the key step in the toluene oxidation. Proton transfer reaction-mass spectrometry (PTR-MS) results revealed the reaction intermediates including benzaldehyde, benzene and phenol, which further decomposed to acetone, ethanol, acetic acid, ketone and acetaldehyde by ring opening before total mineralization. Therefore, PTR-MS provided a facile method to investigate the reaction mechanism of toluene oxidation.
中文翻译:

洞悉易制备的Mn-Cu双金属氧化物催化剂上甲苯全氧化的催化性能和反应路线
在挥发性有机化合物的深度降解中,开发一种简便的制备方法以获得令人满意的过渡金属氧化物基材料的低温催化性能仍然是一个挑战。在此,通过一步水热氧化还原法制备了一系列Mn-Cu双金属氧化物催化剂,用于甲苯的全催化氧化。CH 3 COOH浓度,Cu / Mn摩尔比和煅烧温度极大地影响了晶体结构,微观形貌和催化性能。其中,MnCu尖晶石结构催化剂由于其丰富的表面吸附氧种类,较高的Cu +和Mn 3+含量,因此具有优异的低温催化活性,优异的耐久性和在甲苯全氧化中的耐水性。和优异的低温还原性。在210°C下,MnCu的反应速率比MnCu 0.5的反应速率高7.0倍。具有足够的氧空位的循环氧化还原过程在甲苯氧化中起着至关重要的作用。苯的深度氧化是甲苯氧化的关键步骤。质子转移反应质谱(PTR-MS)结果表明,反应中间体包括苯甲醛,苯和苯酚,它们在完全矿化之前通过开环进一步分解为丙酮,乙醇,乙酸,酮和乙醛。因此,PTR-MS提供了一种简便的方法来研究甲苯氧化的反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号