Frontiers of Environmental Science & Engineering ( IF 6.4 ) Pub Date : 2021-01-26 , DOI: 10.1007/s11783-021-1397-3 Seyyed Salar Meshkat , Ebrahim Ghasemy , Alimorad Rashidi , Omid Tavakoli , Mehdi Esrafili
|
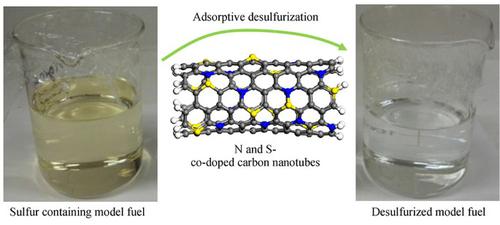
Herein, nitrogen and sulfur co-doped carbon nanotubes (NS-CNT) adsorbents were synthesized via the chemical vapor deposition technique at 1000°C by employing the camphor, urea and sulfur trioxide pyridine. In this study, desulfurization of two types of mercaptans (dibenzothiophene (DBT) and tertiary butyl mercaptan (TBM) as nonlinear and linear forms of mercaptan) was studied. In this regard, a maximum capacity of NS-CNT was obtained as 106.9 and 79.4 mg/g and also the removal efficiencies of 98.6% and 88.3% were achieved after 4 h at 298K and 0.9 g of NS-CNT for DBT and TBM, respectively. Characterization of the NS-CNTs was carried out through exploiting scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and elemental analysis (CHN). The isotherm equilibrium data could be ascribed to the Freundlich nonlinear regression form and the kinetic data was fitted by nonlinear form of the pseudo second order model. The negative values of ΔS0, ΔH0 and ΔG0 specify that the adsorption of both types of mercaptans was a natural exothermic process with a reduced entropy. Maintenance of more than 96% of the adsorption capacity even after nine cycles suggest the NS-CNT as a superior adsorbent for mercaptans removal in the industry. Density functional theory (DFT) calculations were also performed to peruse the effects of S/N co-doping and carbon monovacancy defects in CNTs toward the adsorption of DBT and TBM.
中文翻译:

氮和硫共掺杂碳纳米管对液相有效脱硫的实验和DFT见解:平衡和动力学研究
在此,通过使用樟脑,尿素和三氧化硫吡啶,在1000℃下通过化学气相沉积技术合成了氮和硫共掺杂的碳纳米管(NS-CNT)吸附剂。在这项研究中,研究了两种类型的硫醇(二苯并噻吩(DBT)和叔丁基硫醇(TBM)作为硫醇的非线性和线性形式)的脱硫。在这方面,NS-CNT的最大容量为106.9和79.4 mg / g,并且在298K和0.9 g NS-CNT的DBT和TBM处理4小时后,去除效率分别为98.6%和88.3%,分别。NS-CNT的表征是通过利用扫描电子显微镜(SEM),X射线衍射(XRD),傅里叶变换红外光谱(FTIR)和元素分析(CHN)进行的。等温线平衡数据可以归因于Freundlich非线性回归形式,动力学数据由伪二阶模型的非线性形式拟合。负值ΔS0,ΔH 0和ΔG 0指定两种类型的硫醇的吸附量为具有减小的熵自然放热过程。即使在九个循环后仍保持超过96%的吸附容量,表明NS-CNT是工业上硫醇去除的优良吸附剂。还进行了密度泛函理论(DFT)计算,以研究S / N共掺杂和CNT中碳单空位缺陷对DBT和TBM吸附的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号