当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Asymmetric Difluoro‐Reformatsky Reaction
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-01-29 , DOI: 10.1002/ejoc.202100004 Manfred Braun 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-01-29 , DOI: 10.1002/ejoc.202100004 Manfred Braun 1
Affiliation
|
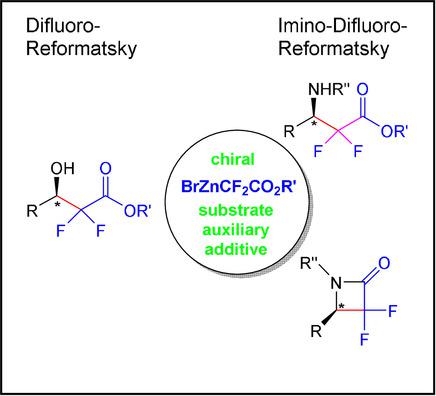
Convenient, versatile, reliable, and functional‐group tolerant: The Reformatsky reaction! Over 130 years old, it is still a good choice when synthesis requires the introduction of the difluoroacetate enolate into aldehydes and imines. Appropriately chosen chiral substrates, auxiliaries, or additives enable the creation of the new stereogenic center and make asymmetric syntheses of difluorinated biologically active targets accessible.

中文翻译:

不对称二氟Reformatsky反应
方便,通用,可靠和功能组兼容:Reformatsky的反应!已有130多年的历史了,当合成需要将二氟乙酸烯醇酯引入醛和亚胺中时,它仍然是一个不错的选择。选择适当的手性底物,助剂,添加剂或启用新的立体中心的创作和制作的二氟化的生物活性指标不对称合成访问。

更新日期:2021-03-23

中文翻译:

不对称二氟Reformatsky反应
方便,通用,可靠和功能组兼容:Reformatsky的反应!已有130多年的历史了,当合成需要将二氟乙酸烯醇酯引入醛和亚胺中时,它仍然是一个不错的选择。选择适当的手性底物,助剂,添加剂或启用新的立体中心的创作和制作的二氟化的生物活性指标不对称合成访问。












































 京公网安备 11010802027423号
京公网安备 11010802027423号