Solid State Ionics ( IF 3.0 ) Pub Date : 2021-01-28 , DOI: 10.1016/j.ssi.2021.115566 Yasumasa Tomita , Ryo Saito , Makoto Morishita , Yohei Yamane , Yoshiumi Kohno
|
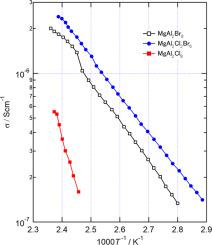
MgAl2X8 (X = Cl, Br) was synthesized from MgX2 and AlX3, and the physical properties were evaluated by XRD, AC conductivity, and NMR. MgAl2Br8 has the same crystal structure as MgAl2Cl8 and belongs to the space group C2/c. MgAl2Cl8-yBry was also synthesized in the same manner as MgAl2Br8. MgAl2Cl8-yBry was found to have the same crystal structure as MgAl2Cl8 and forms a solid solution in all the synthesized compositions. From the Rietveld analysis of the XRD pattern, it was found that the arrangement of Cl and Br in the crystal had no order and occupied the halogen sites almost randomly. The AC conductivity of the obtained compound was lowest for MgAl2Cl8 and highest for MgAl2Cl2Br6. The conductivity of MgAl2Cl2Br6 at 400 K is 1.3 × 10−6 S/cm, and the Nyquist plot suggests that MgAl2Cl8-yBry is a magnesium ionic conductor. In the temperature range from room temperature to 412 K, there was no significant change in the 27Al NMR spectra of a MgAl2Br8 single crystal, and the effects of Mg2+ ionic conduction and the reorientation motion of AlX4ˉ anion were not observed.
中文翻译:

MgAl 2 X 8(X = Cl,Br)的合成,晶体结构和离子电导率
由MgX 2和AlX 3合成MgAl 2 X 8(X = Cl,Br),并通过XRD,AC电导率和NMR评估其物理性质。MgAl 2 Br 8具有与MgAl 2 Cl 8相同的晶体结构,并且属于空间群C2 / c。还以与MgAl 2 Br 8相同的方式合成了MgAl 2 Cl 8-y Br y。发现MgAl 2 Cl 8-y Br y具有与MgAl 2 Cl 8相同的晶体结构。并在所有合成的组合物中形成固溶体。从XRD图的Rietveld分析,发现Cl和Br在晶体中的排列无序并且几乎随机地占据卤素位点。所得化合物的AC电导率对于MgAl 2 Cl 8最低,而对于MgAl 2 Cl 2 Br 6最高。MgAl 2 Cl 2 Br 6在400 K下的电导率为1.3×10 -6 S / cm,奈奎斯特图表明MgAl 2 Cl 8-y Br y是镁离子导体。在温度范围为室温至412 K,有一个在没有显著变化27一个的MgAl的铝NMR谱2溴8单晶和Mg的效果2+离子传导和ALX的重新定向运动4 ˉ阴离子是没有观察到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号