当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomically dispersed nonmagnetic electron traps improve oxygen reduction activity of perovskite oxides
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-1-22 , DOI: 10.1039/d0ee03701j Zechao Zhuang 1, 2, 3, 4 , Yong Li 5, 6, 7, 8 , Yihang Li 4, 9, 10, 11 , Jiazhao Huang 4, 12, 13, 14, 15 , Bin Wei 16, 17, 18 , Rong Sun 16, 17, 18 , Yujing Ren 19, 20, 21, 22, 23 , Jie Ding 19, 20, 21, 22, 23 , Jiexin Zhu 4, 15, 24, 25 , Zhiquan Lang 4, 26, 27, 28, 29 , Lyudmila V. Moskaleva 5, 6, 7, 8 , Chuanxin He 4, 9, 10, 11 , Yu Wang 4, 22, 30, 31, 32 , Zhongchang Wang 16, 17, 18 , Dingsheng Wang 1, 2, 3, 4 , Yadong Li 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-1-22 , DOI: 10.1039/d0ee03701j Zechao Zhuang 1, 2, 3, 4 , Yong Li 5, 6, 7, 8 , Yihang Li 4, 9, 10, 11 , Jiazhao Huang 4, 12, 13, 14, 15 , Bin Wei 16, 17, 18 , Rong Sun 16, 17, 18 , Yujing Ren 19, 20, 21, 22, 23 , Jie Ding 19, 20, 21, 22, 23 , Jiexin Zhu 4, 15, 24, 25 , Zhiquan Lang 4, 26, 27, 28, 29 , Lyudmila V. Moskaleva 5, 6, 7, 8 , Chuanxin He 4, 9, 10, 11 , Yu Wang 4, 22, 30, 31, 32 , Zhongchang Wang 16, 17, 18 , Dingsheng Wang 1, 2, 3, 4 , Yadong Li 1, 2, 3, 4
Affiliation
|
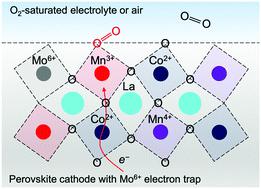
Complexity in strongly correlated oxides such as perovskite strictly dominates their performance for oxygen reduction reaction (ORR). Precise control of the physical correlations among spin, charge, orbital, and lattice degrees of freedom in these oxides can exercise considerable enhancement of ORR activity, but has until now remained elusive. Here, we show that nonmagnetic hexavalent molybdenum (Mo6+) atomically dispersed within oxide lattice steers the intrinsic activity of catalytically active sites by entrapping extrinsic electrons at their 3d orbitals, without the occurrence of lattice symmetry breaking and magnetic perturbation. With double perovskite La2Co2+Mn4+O6 as a model catalyst, the atomic-scale electron trap generates additional high-spin, catalytically active Mn3+(t32ge1g) sites and highly conductive Co2+(e2g)–O–Mn3+(e1g) double exchange channels, leading to five-fold improvement in ORR activity. First-principles calculations reveal a substantial increase of the spin density on Mn sites caused by electron trapping, and unambiguously confirm a more exothermic reaction pathway as well as a lower barrier of the rate-limiting surface hydroxide regeneration on Mo1/La2CoMnO6. We can also extend this strategy with atomic precision easily to other four oxide catalysts and achieve large enhancement in their ORR activities as anticipated, indicating its broad utility. This work embodies the theories of condensed matter physics in rational design of ORR catalysts, and may inspire further development of the control of electron correlation in strongly correlated electron systems.
中文翻译:

原子分散的非磁性电子陷阱可提高钙钛矿氧化物的氧还原活性
钙钛矿等强相关氧化物中的复杂性严格控制了其氧还原反应(ORR)的性能。精确控制这些氧化物中自旋,电荷,轨道和晶格自由度之间的物理相关性可以显着提高ORR活性,但是迄今为止仍然难以捉摸。在这里,我们表明原子分散在氧化物晶格中的非磁性六价钼(Mo 6+)通过在3d轨道上捕获外在电子来控制催化活性位的内在活性,而没有发生晶格对称性破坏和磁扰动。含双钙钛矿La 2 Co 2+ Mn 4+ O 6作为模型催化剂,原子级电子陷阱产生额外的高自旋,催化活性的Mn 3+(t 3 2g e 1 g)位和高导电性Co 2+(e 2 g)–O–Mn 3+(e 1 g)双交换通道,导致ORR活性提高了五倍。第一性原理计算表明,由于电子俘获,Mn位上的自旋密度显着增加,并且明确证实了更多的放热反应路径以及对Mo 1 / La 2 CoMnO 6的限速表面氢氧化物再生的较低障碍。。我们还可以轻松地将这种策略以原子精度轻松地扩展到其他四种氧化物催化剂,并实现其ORR活性的大幅提高(如预期的那样),表明其广泛的用途。这项工作在ORR催化剂的合理设计中体现了凝聚态物理学的理论,并可能激发强相关电子系统中电子相关控制的进一步发展。
更新日期:2021-01-28
中文翻译:

原子分散的非磁性电子陷阱可提高钙钛矿氧化物的氧还原活性
钙钛矿等强相关氧化物中的复杂性严格控制了其氧还原反应(ORR)的性能。精确控制这些氧化物中自旋,电荷,轨道和晶格自由度之间的物理相关性可以显着提高ORR活性,但是迄今为止仍然难以捉摸。在这里,我们表明原子分散在氧化物晶格中的非磁性六价钼(Mo 6+)通过在3d轨道上捕获外在电子来控制催化活性位的内在活性,而没有发生晶格对称性破坏和磁扰动。含双钙钛矿La 2 Co 2+ Mn 4+ O 6作为模型催化剂,原子级电子陷阱产生额外的高自旋,催化活性的Mn 3+(t 3 2g e 1 g)位和高导电性Co 2+(e 2 g)–O–Mn 3+(e 1 g)双交换通道,导致ORR活性提高了五倍。第一性原理计算表明,由于电子俘获,Mn位上的自旋密度显着增加,并且明确证实了更多的放热反应路径以及对Mo 1 / La 2 CoMnO 6的限速表面氢氧化物再生的较低障碍。。我们还可以轻松地将这种策略以原子精度轻松地扩展到其他四种氧化物催化剂,并实现其ORR活性的大幅提高(如预期的那样),表明其广泛的用途。这项工作在ORR催化剂的合理设计中体现了凝聚态物理学的理论,并可能激发强相关电子系统中电子相关控制的进一步发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号