当前位置:
X-MOL 学术
›
Environ. Toxicol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concentration Addition, Independent Action, and Quantitative Structure–Activity Relationships for Chemical Mixture Toxicities of the Disinfection By products of Haloacetic Acids on the Green Alga Raphidocelis subcapitata
Environmental Toxicology and Chemistry ( IF 3.6 ) Pub Date : 2021-01-28 , DOI: 10.1002/etc.4995 Li-Tang Qin 1, 2, 3 , Min Liu 1 , Xin Zhang 1 , Ling-Yun Mo 3, 4 , Hong-Hu Zeng 1, 2, 4 , Yan-Peng Liang 1, 2, 4
Environmental Toxicology and Chemistry ( IF 3.6 ) Pub Date : 2021-01-28 , DOI: 10.1002/etc.4995 Li-Tang Qin 1, 2, 3 , Min Liu 1 , Xin Zhang 1 , Ling-Yun Mo 3, 4 , Hong-Hu Zeng 1, 2, 4 , Yan-Peng Liang 1, 2, 4
Affiliation
|
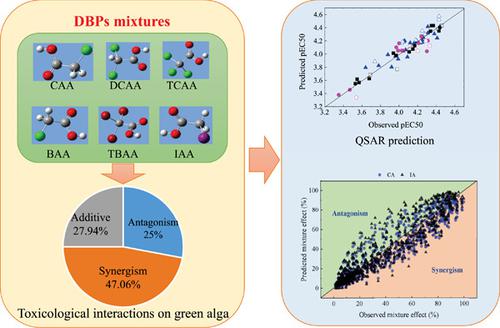
The potential toxicity of haloacetic acids (HAAs), common disinfection by products (DBPs), has been widely studied; but their combined effects on freshwater green algae remain poorly understood. The present study was conducted to investigate the toxicological interactions of HAA mixtures in the green alga Raphidocelis subcapitata and predict the DBP mixture toxicities based on concentration addition, independent action, and quantitative structure–activity relationship (QSAR) models. The acute toxicities of 6 HAAs (iodoacetic acid [IAA], bromoacetic acid [BAA], chloroacetic acid [CAA], dichloroacetic acid [DCAA], trichloroacetic acid [TCAA], and tribromoacetic acid [TBAA]) and their 68 binary mixtures to the green algae were analyzed in 96‐well microplates. Results reveal that the rank order of the toxicity of individual HAAs is CAA > IAA ≈ BAA > TCAA > DCAA > TBAA. With concentration addition as the reference additive model, the mixture effects are synergetic in 47.1% and antagonistic in 25%, whereas the additive effects are only observed in 27.9% of the experiments. The main components that induce synergism are DCAA, IAA, and BAA; and CAA is the main component that causes antagonism. Prediction by concentration addition and independent action indicates that the 2 models fail to accurately predict 72% mixture toxicity at an effective concentration level of 50%. Modeling the mixtures by QSAR was established by statistically analyzing descriptors for the determination of the relationship between their chemical structures and the negative logarithm of the 50% effective concentration. The additive mixture toxicities are accurately predicted by the QSAR model based on 2 parameters, the octanol–water partition coefficient and the acid dissociation constant (pKa). The toxicities of synergetic mixtures can be interpreted with the total energy (ET) and pKa of the mixtures. Dipole moment and ET are the quantum descriptors that influence the antagonistic mixture toxicity. Therefore, in silico modeling may be a useful tool in predicting disinfection by‐product mixture toxicities. Environ Toxicol Chem 2021;40:1431–1442. © 2021 SETAC
中文翻译:

卤代乙酸消毒副产物对绿藻Raphidocelis subcapitata 的化学混合物毒性的浓度加成、独立作用和定量结构-活性关系
卤代乙酸 (HAA) 是常见的消毒副产物 (DBP),其潜在毒性已被广泛研究;但它们对淡水绿藻的综合影响仍然知之甚少。本研究旨在调查 HAA 混合物在绿藻Raphidocelis subcapitata 中的毒理学相互作用并根据浓度添加、独立作用和定量构效关系 (QSAR) 模型预测 DBP 混合物的毒性。6 种 HAA(碘乙酸 [IAA]、溴乙酸 [BAA]、氯乙酸 [CAA]、二氯乙酸 [DCAA]、三氯乙酸 [TCAA] 和三溴乙酸 [TBAA])及其 68 种二元混合物的急性毒性在 96 孔微孔板中分析绿藻。结果表明,单个HAA的毒性排序为CAA>IAA≈BAA>TCAA>DCAA>TBAA。以浓度加法作为参考加法模型,混合效应在 47.1% 的情况下是协同的,在 25% 的情况下是拮抗的,而加性效应仅在 27.9% 的实验中观察到。引起协同作用的主要成分是 DCAA、IAA 和 BAA;而CAA是引起拮抗作用的主要成分。通过浓度相加和独立作用的预测表明,在 50% 的有效浓度水平下,这 2 个模型未能准确预测 72% 的混合物毒性。用 QSAR 对混合物进行建模是通过统计分析描述符来建立的,以确定其化学结构与 50% 有效浓度的负对数之间的关系。QSAR 模型基于 2 个参数、辛醇-水分配系数和酸解离常数 (p 用 QSAR 对混合物进行建模是通过统计分析描述符来建立的,以确定其化学结构与 50% 有效浓度的负对数之间的关系。QSAR 模型基于 2 个参数、辛醇-水分配系数和酸解离常数 (p 用 QSAR 对混合物进行建模是通过统计分析描述符来建立的,以确定其化学结构与 50% 有效浓度的负对数之间的关系。QSAR 模型基于 2 个参数、辛醇-水分配系数和酸解离常数 (pK a )。协同混合物的毒性可以用混合物的总能量 ( E T ) 和 p K a来解释。偶极矩和E T是影响拮抗混合物毒性的量子描述符。因此,计算机模拟可能是预测消毒副产物混合物毒性的有用工具。环境毒理学化学2021;40:1431-1442。© 2021 SETAC
更新日期:2021-01-28
中文翻译:

卤代乙酸消毒副产物对绿藻Raphidocelis subcapitata 的化学混合物毒性的浓度加成、独立作用和定量结构-活性关系
卤代乙酸 (HAA) 是常见的消毒副产物 (DBP),其潜在毒性已被广泛研究;但它们对淡水绿藻的综合影响仍然知之甚少。本研究旨在调查 HAA 混合物在绿藻Raphidocelis subcapitata 中的毒理学相互作用并根据浓度添加、独立作用和定量构效关系 (QSAR) 模型预测 DBP 混合物的毒性。6 种 HAA(碘乙酸 [IAA]、溴乙酸 [BAA]、氯乙酸 [CAA]、二氯乙酸 [DCAA]、三氯乙酸 [TCAA] 和三溴乙酸 [TBAA])及其 68 种二元混合物的急性毒性在 96 孔微孔板中分析绿藻。结果表明,单个HAA的毒性排序为CAA>IAA≈BAA>TCAA>DCAA>TBAA。以浓度加法作为参考加法模型,混合效应在 47.1% 的情况下是协同的,在 25% 的情况下是拮抗的,而加性效应仅在 27.9% 的实验中观察到。引起协同作用的主要成分是 DCAA、IAA 和 BAA;而CAA是引起拮抗作用的主要成分。通过浓度相加和独立作用的预测表明,在 50% 的有效浓度水平下,这 2 个模型未能准确预测 72% 的混合物毒性。用 QSAR 对混合物进行建模是通过统计分析描述符来建立的,以确定其化学结构与 50% 有效浓度的负对数之间的关系。QSAR 模型基于 2 个参数、辛醇-水分配系数和酸解离常数 (p 用 QSAR 对混合物进行建模是通过统计分析描述符来建立的,以确定其化学结构与 50% 有效浓度的负对数之间的关系。QSAR 模型基于 2 个参数、辛醇-水分配系数和酸解离常数 (p 用 QSAR 对混合物进行建模是通过统计分析描述符来建立的,以确定其化学结构与 50% 有效浓度的负对数之间的关系。QSAR 模型基于 2 个参数、辛醇-水分配系数和酸解离常数 (pK a )。协同混合物的毒性可以用混合物的总能量 ( E T ) 和 p K a来解释。偶极矩和E T是影响拮抗混合物毒性的量子描述符。因此,计算机模拟可能是预测消毒副产物混合物毒性的有用工具。环境毒理学化学2021;40:1431-1442。© 2021 SETAC











































 京公网安备 11010802027423号
京公网安备 11010802027423号