当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Assessment of SARS-CoV-2 Virus in Nasopharyngeal Swabs Stored in Transport Medium by a Straightforward LC-MS/MS Assay Targeting Nucleocapsid, Membrane, and Spike Proteins
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2021-01-26 , DOI: 10.1021/acs.jproteome.0c00887 Justyna Saadi 1 , Saoussen Oueslati 2 , Laurent Bellanger 3 , Fabrice Gallais 3 , Laurent Dortet 2 , Anne-Marie Roque-Afonso 4 , Christophe Junot 1 , Thierry Naas 2 , François Fenaille 1 , François Becher 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2021-01-26 , DOI: 10.1021/acs.jproteome.0c00887 Justyna Saadi 1 , Saoussen Oueslati 2 , Laurent Bellanger 3 , Fabrice Gallais 3 , Laurent Dortet 2 , Anne-Marie Roque-Afonso 4 , Christophe Junot 1 , Thierry Naas 2 , François Fenaille 1 , François Becher 1
Affiliation
|
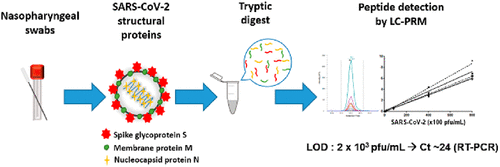
Alternative methods to RT-PCR for SARS-CoV-2 detection are investigated to provide complementary data on viral proteins, increase the number of tests performed, or identify false positive/negative results. Here, we have developed a simple mass spectrometry assay for SARS-CoV-2 in nasopharyngeal swab samples using common laboratory reagents. The method employs high sensitivity and selectivity targeted mass spectrometry detection, monitoring nine constitutive peptides representative of the three main viral proteins and a straightforward pellet digestion protocol for convenient routine applications. Absolute quantification of N, M, and S proteins was achieved by addition of isotope-labeled versions of best peptides. Limit of detection, recovery, precision, and linearity were thoroughly evaluated in four representative viral transport media (VTM) containing distinct total protein content. The protocol was sensitive in all swab media with limit of detection determined at 2 × 103 pfu/mL, corresponding to as low as 30 pfu injected into the LC-MS/MS system. When tested on VTM-stored nasopharyngeal swab samples from positive and control patients, sensitivity was similar to or better than rapid immunoassay dipsticks, revealing a corresponding RT-PCR detection threshold at Ct ∼ 24. The study represents the first thorough evaluation of sensitivity and robustness of targeted mass spectrometry in nasal swabs, constituting a promising SARS-CoV-2 antigen assay for the first-line diagnosis of COVID-19 and compatible with the constraints of clinical settings. The raw files generated in this study can be found on PASSEL (Peptide Atlas) under data set identifier PASS01646.
中文翻译:

通过直接针对核衣壳,膜和穗蛋白的LC-MS / MS分析定量评估存储在运输介质中的鼻咽拭子中的SARS-CoV-2病毒
研究了用于SARS-CoV-2检测的RT-PCR替代方法,以提供有关病毒蛋白的补充数据,增加检测次数或识别假阳性/阴性结果。在这里,我们开发了一种使用常规实验室试剂对鼻咽拭子样品中的SARS-CoV-2进行简单质谱分析的方法。该方法采用高灵敏度和选择性的靶向质谱检测,监测代表三种主要病毒蛋白的九种组成性肽以及用于常规常规应用的直接沉淀消化方案。N,M和S蛋白的绝对定量是通过添加同位素标记的最佳肽形式实现的。检测极限,恢复精度 在包含不同总蛋白质含量的四种代表性病毒运输介质(VTM)中,对线性和线性进行了全面评估。该方案在所有拭子培养基中均敏感,检测极限确定为2×103 pfu / mL,相当于注入到LC-MS / MS系统中的低至30 pfu。当对阳性和对照患者的VTM鼻咽拭子样本进行测试时,敏感性与快速免疫检测试纸相似或更好,揭示了相应的RT-PCR检测阈值Ct〜24。该研究代表了对敏感性和鲁棒性的首次全面评估。鼻拭子靶向质谱分析技术的成功应用,构成了用于COVID-19一线诊断的有前途的SARS-CoV-2抗原检测方法,并且与临床环境兼容。可以在PASSEL(肽图集)上以数据集标识符PASS01646找到该研究中生成的原始文件。
更新日期:2021-02-05
中文翻译:

通过直接针对核衣壳,膜和穗蛋白的LC-MS / MS分析定量评估存储在运输介质中的鼻咽拭子中的SARS-CoV-2病毒
研究了用于SARS-CoV-2检测的RT-PCR替代方法,以提供有关病毒蛋白的补充数据,增加检测次数或识别假阳性/阴性结果。在这里,我们开发了一种使用常规实验室试剂对鼻咽拭子样品中的SARS-CoV-2进行简单质谱分析的方法。该方法采用高灵敏度和选择性的靶向质谱检测,监测代表三种主要病毒蛋白的九种组成性肽以及用于常规常规应用的直接沉淀消化方案。N,M和S蛋白的绝对定量是通过添加同位素标记的最佳肽形式实现的。检测极限,恢复精度 在包含不同总蛋白质含量的四种代表性病毒运输介质(VTM)中,对线性和线性进行了全面评估。该方案在所有拭子培养基中均敏感,检测极限确定为2×103 pfu / mL,相当于注入到LC-MS / MS系统中的低至30 pfu。当对阳性和对照患者的VTM鼻咽拭子样本进行测试时,敏感性与快速免疫检测试纸相似或更好,揭示了相应的RT-PCR检测阈值Ct〜24。该研究代表了对敏感性和鲁棒性的首次全面评估。鼻拭子靶向质谱分析技术的成功应用,构成了用于COVID-19一线诊断的有前途的SARS-CoV-2抗原检测方法,并且与临床环境兼容。可以在PASSEL(肽图集)上以数据集标识符PASS01646找到该研究中生成的原始文件。







































 京公网安备 11010802027423号
京公网安备 11010802027423号