当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Forging C‐SeCF3 Bonds with Trifluoromethyl Tolueneselenosulfonate under Visible‐Light
The Chemical Record ( IF 7.0 ) Pub Date : 2021-01-27 , DOI: 10.1002/tcr.202000184 Dan Louvel 1 , Clément Ghiazza 1 , Vincent Debrauwer 1 , Lhoussain Khrouz 2 , Cyrille Monnereau 2 , Anis Tlili 1
The Chemical Record ( IF 7.0 ) Pub Date : 2021-01-27 , DOI: 10.1002/tcr.202000184 Dan Louvel 1 , Clément Ghiazza 1 , Vincent Debrauwer 1 , Lhoussain Khrouz 2 , Cyrille Monnereau 2 , Anis Tlili 1
Affiliation
|
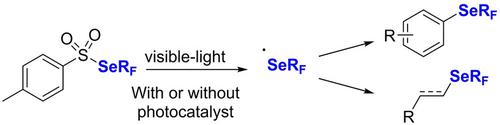
This account highlights some of our recent work on photoinduced trifluoromethylselenolation reactions. This research program relies primarily on the design of a new key shelf‐stable selenating reagent that can be involved in various radical processes In particular, we demonstrated that trifluoromethylselenolation of arenes, alkenes, alkynes as well as aliphatic organic building blocks can be readily achieved under visible‐light irradiation. Mechanistic investigations based on 19F NMR studies, EPR spectroscopy, cyclic voltammetry and luminescence studies allowed us to shed the light on the different proposed mechanisms in the designed methodologies. The applicative potential of these strategies was further demonstrated through the synthesis of bioactive analogue containing SeCF3 motif.
中文翻译:

在可见光下用三氟甲基甲苯磺酸盐锻造C-SeCF3键
此说明重点介绍了我们最近在光诱导的三氟甲基硒代反应方面的一些工作。该研究计划主要依赖于可参与各种自由基过程的新型关键货架稳定硒化试剂的设计。特别是,我们证明了在以下条件下,芳烃,烯烃,炔烃和脂肪族有机结构单元的三氟甲基硒化反应很容易实现。可见光照射。基于19 F NMR研究,EPR光谱学,循环伏安法和发光研究的机理研究使我们得以了解设计方法学中提出的不同机制。通过合成含有SeCF 3基序的生物活性类似物,进一步证明了这些策略的应用潜力。
更新日期:2021-02-18
中文翻译:

在可见光下用三氟甲基甲苯磺酸盐锻造C-SeCF3键
此说明重点介绍了我们最近在光诱导的三氟甲基硒代反应方面的一些工作。该研究计划主要依赖于可参与各种自由基过程的新型关键货架稳定硒化试剂的设计。特别是,我们证明了在以下条件下,芳烃,烯烃,炔烃和脂肪族有机结构单元的三氟甲基硒化反应很容易实现。可见光照射。基于19 F NMR研究,EPR光谱学,循环伏安法和发光研究的机理研究使我们得以了解设计方法学中提出的不同机制。通过合成含有SeCF 3基序的生物活性类似物,进一步证明了这些策略的应用潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号