当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, structural and DFT investigation of Zn(nba)2(meim)2 for adsorptive removal of eosin yellow dye from aqueous solution
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2021-01-27 , DOI: 10.1002/zaac.202000425 Adetola Oladipo 1 , Adedibu C. Tella 2 , Victoria T. Olayemi 3 , Vincent O. Adimula 2 , Tendai O. Dembaremba 4 , Adeniyi S. Ogunlaja 4 , Hadley S. Clayton 5 , Guy J. Clarkson 6 , Richard I. Walton 6
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2021-01-27 , DOI: 10.1002/zaac.202000425 Adetola Oladipo 1 , Adedibu C. Tella 2 , Victoria T. Olayemi 3 , Vincent O. Adimula 2 , Tendai O. Dembaremba 4 , Adeniyi S. Ogunlaja 4 , Hadley S. Clayton 5 , Guy J. Clarkson 6 , Richard I. Walton 6
Affiliation
|
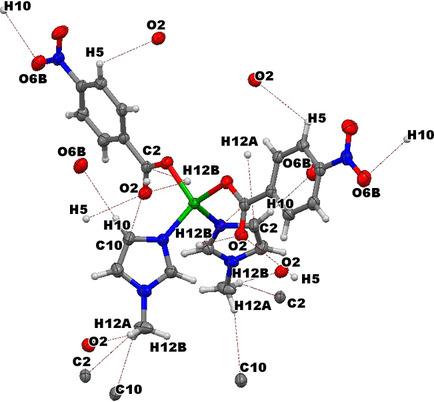
A novel Zn(II) mixed‐ligand complex, Zn(nba)2(meim)2 (1), synthesized from Zn(NO3)2.6H2O, nitrobenzoic acid (Hnba) and 1‐methylimidazole (meim) is reported. The complex was characterized by elemental analysis, FT‐IR, powder and single crystal X‐ray crystallography and TGA/DSC. 1 exhibits a tetrahedral geometry for Zn(II), which is coordinated to two carboxylate oxygen atoms from two nba anions and two imidazole nitrogen atoms from two meim molecules. Thermal analysis shows the stability of 1 up to 260 °C. The adsorption of eosin yellow (EY) dye on 1 was investigated. The adsorption capacity of 1 for EY amounted to 65.32 mg/g, fitting best into Langmuir isotherm and pseudo second order kinetic models. From DFT studies, it was determined that adsorption is predominantly due to electrostatic, hydrogen bonding and π‐π interactions.
中文翻译:

Zn(nba)2(meim)2的合成,结构和DFT研究用于从水溶液中吸附去除曙红黄色染料
一种新型的锌(II)的混合配体配合物,锌(NBA)2(MEIM)2(1)中,从合成的Zn(NO 3)2 ·6H 2 O,硝基苯甲酸(Hnba)和1-甲基咪唑(MEIM)是报告。该复合物通过元素分析,FT-IR,粉末和单晶X射线晶体学以及TGA / DSC进行表征。图1显示了Zn(II)的四面体几何结构,它与来自两个nba阴离子的两个羧酸氧原子和来自两个meim分子的两个咪唑氮原子配位。热分析表明在高达260°C的温度下具有1的稳定性。研究了曙红黄(EY)染料在1上的吸附。的吸附能力EY的1为65.32 mg / g,最适合Langmuir等温线和伪二级动力学模型。根据DFT研究,可以确定吸附主要是由于静电,氢键和π-π相互作用引起的。
更新日期:2021-04-02
中文翻译:

Zn(nba)2(meim)2的合成,结构和DFT研究用于从水溶液中吸附去除曙红黄色染料
一种新型的锌(II)的混合配体配合物,锌(NBA)2(MEIM)2(1)中,从合成的Zn(NO 3)2 ·6H 2 O,硝基苯甲酸(Hnba)和1-甲基咪唑(MEIM)是报告。该复合物通过元素分析,FT-IR,粉末和单晶X射线晶体学以及TGA / DSC进行表征。图1显示了Zn(II)的四面体几何结构,它与来自两个nba阴离子的两个羧酸氧原子和来自两个meim分子的两个咪唑氮原子配位。热分析表明在高达260°C的温度下具有1的稳定性。研究了曙红黄(EY)染料在1上的吸附。的吸附能力EY的1为65.32 mg / g,最适合Langmuir等温线和伪二级动力学模型。根据DFT研究,可以确定吸附主要是由于静电,氢键和π-π相互作用引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号