Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-01-27 , DOI: 10.1016/j.jece.2021.105125 Veer Singh , Jyoti Singh , Vishal Mishra
|
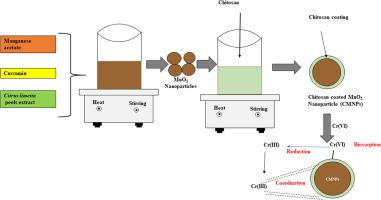
In the present work, Cr (VI) was successfully biosorbed and reduced to less toxic Cr (III) by using chitosan coated MnO2 nanoparticles synthesized through green route using curcumin and Citrus limetta peel extract. X-ray photoelectron spectroscopic analysis of chromium loaded nanoparticles confirmed that most of the Cr (VI) ions were reduced to Cr (III). Scanning electron and transmission electron microscopic images showed that these nanoparticles were spherical in shape, crystalline in nature, ranged from 14 to 24 nm in size. The Fourier-transform infrared spectroscopy analysis indicated hydroxyl, carboxyl and amino groups on its surface. The dynamic light scattering showed a size distribution of nanoparticles as 299.50 ± 25.32 nm. High Brunauer- Emmett-Teller specific surface area (76.19 m2/g) of these nanoparticles provided more active sites for binding of chromium ions. Energy dispersive X-ray and ultimate analysis indicated the presence of Mn, O, C, H, and N. These elements were the major components of these nanoparticles and confirmed the presence of chitosan on nanoparticle surface. The lower value of pHzpc (3.17) proved the suitability of acidic pH values for conducting biosorption experiments. The values of dimensionless numbers and diffusivity coefficients indicated that biosorption was controlled by film and pore diffusion. Pseudo-second order (R2 = 0.99) and Freundlich isotherm (R2 = 0.99) showed a better goodness of fit over other kinetic and isotherm models. The thermodynamic study showed that biosorption was exothermic and spontaneous. But, the enthalpy is endothermic and the reaction is entropy driven. Results showed that these nanoparticles have high Cr (VI) uptake capacity (92.19 mg/g) and can be reused over several cycles of adsorption–desorption.
中文翻译:

间歇式反应器中环保且可持续的Cr(VI)离子清除剂的吸附动力学
在目前的工作中,使用姜黄素和柑桔通过绿色途径合成的壳聚糖包被的MnO 2纳米粒子通过绿色途径合成,成功地吸附了六价铬(Cr),并将其还原为毒性较小的三价铬(III)。果皮提取物。铬负载纳米粒子的X射线光电子能谱分析证实,大多数Cr(VI)离子都还原为Cr(III)。扫描电子显微镜和透射电子显微镜图像显示,这些纳米颗粒为球形,本质上为晶体,尺寸为14至24nm。傅立叶变换红外光谱分析表明其表面上有羟基,羧基和氨基。动态光散射显示纳米颗粒的尺寸分布为299.50±25.32nm。高布鲁诺尔-埃默特-泰勒比表面积(76.19 m 2/ g)这些纳米颗粒为结合铬离子提供了更多的活性位点。能量色散X射线和最终分析表明存在Mn,O,C,H和N。这些元素是这些纳米粒子的主要成分,并证实了纳米粒子表面存在壳聚糖。pH zpc(3.17)的较低值证明了酸性pH值适合进行生物吸附实验。无量纲数和扩散系数的值表明生物吸附受膜和孔扩散的控制。伪二阶(R 2 = 0.99)和Freundlich等温线(R 2= 0.99)显示出比其他动力学和等温线模型更好的拟合优度。热力学研究表明生物吸附是放热的并且是自发的。但是,焓是吸热的,反应是熵驱动的。结果表明,这些纳米颗粒具有较高的Cr(VI)吸收能力(92.19 mg / g),可以在多个吸附-解吸循环中重复使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号