当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complying with the physiological functions of Golgi apparatus for secretory exocytosis facilitated oral absorption of protein drugs
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0tb02848g Liyun Xing 1, 2, 3, 4, 5 , Yaxian Zheng 1, 2, 3, 4, 5 , Yinglan Yu 1, 2, 3, 4, 5 , Ruinan Wu 1, 2, 3, 4, 5 , Xi Liu 1, 2, 3, 4, 5 , Rui Zhou 1, 2, 3, 4, 5 , Yuan Huang 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0tb02848g Liyun Xing 1, 2, 3, 4, 5 , Yaxian Zheng 1, 2, 3, 4, 5 , Yinglan Yu 1, 2, 3, 4, 5 , Ruinan Wu 1, 2, 3, 4, 5 , Xi Liu 1, 2, 3, 4, 5 , Rui Zhou 1, 2, 3, 4, 5 , Yuan Huang 1, 2, 3, 4, 5
Affiliation
|
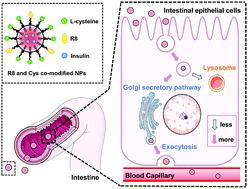
Intestinal epithelial cells are the primary biological barriers for orally administrated nano-formulations and the delivered protein drugs. Thereinto, besides the cellular uptake, intracellular trafficking pathway and the related exocytosis are of great importance to the trans-epithelial transport of drug-loaded NPs. Herein, inspired by the physiological functions of Golgi apparatus for secreting proteins out of cells, Golgi localization-related amino acid L-cysteine (Cys) was modified on the surface of NPs to see whether and how this modification could guide the Golgi pathway-related transport and facilitate the exocytosis of drug-loaded NPs. Meanwhile, cell-penetrating peptide octa-arginine (R8) was co-modified to increase the cellular uptake. The proportion of R8 and Cys modification was explored to get the best effect of endocytosis and exocytosis of NPs. As a result, 25%R8 + 75%Cys NPs with most Cys modification showed efficient transcytosis with the highest transcytosis/endocytosis ratio (0.87). Interestingly, exocytosis mechanism studies indicated that they trafficked through the Golgi secretory pathway and bypassed lysosomes due to Cys modification. The detailed Golgi position mechanism studies further suggested that the thiol group from Cys was important for mediating Golgi transport. In particular, competitive inhibition studies demonstrated that Cys-modified NPs were more conducive to their exocytosis after being transported through the Golgi secretory pathway. We proved that cargos transported via Golgi apparatus tended to be trafficked out of the cells and avoid degradation, which contributed to the transcytosis of 25%R8 + 75%Cys NPs in vitro. Inspiringly, compared with unmodified NPs, 25%R8 + 75%Cys NPs also exhibited promoted intestinal penetration and oral absorption in vivo. Oral delivery of insulin-loaded 25%R8 + 75%Cys NPs showed stronger hypoglycemic effects in diabetic rats. In summary, this work provides a strategy for complying with the physiological functions of Golgi apparatus for secreting to facilitate the exocytosis of NPs, thus further improving the oral absorption of loaded protein drugs.
中文翻译:

符合高尔基体对分泌性胞吐作用的生理功能,促进蛋白质药物的口服吸收
肠上皮细胞是口服纳米制剂和所输送蛋白药物的主要生物屏障。其中,除了细胞摄取外,细胞内运输途径和相关的胞吐作用对于载有药物的NP的跨上皮运输非常重要。在此,受高尔基体从细胞分泌蛋白质的生理功能启发,高尔基体定位相关氨基酸L-半胱氨酸(Cys)在NP的表面进行了修饰,以查看这种修饰是否以及如何指导高尔基途径相关的运输并促进载药NP的胞吐作用。同时,将穿透细胞的肽八精氨酸(R8)共修饰以增加细胞摄取。探索了R8和Cys修饰的比例,以获得NP的内吞作用和胞吐作用的最佳效果。结果,具有最多Cys修饰的25%R8 + 75%Cys NPs表现出高效的胞吞作用,具有最高的胞吞作用/胞吞作用比率(0.87)。有趣的是,胞吐机制研究表明,由于Cys修饰,它们通过高尔基体分泌途径贩运并绕过了溶酶体。详细的高尔基体定位机制研究进一步表明,来自Cys的巯基对介导高尔基体运输很重要。特别地,竞争性抑制研究表明,Cys修饰的NP在通过高尔基体分泌途径转运后,更有助于其胞吐作用。我们证明货物已经运输通过高尔基体的装置倾向于从细胞中运出并避免降解,这有助于体外25%R8 + 75%Cys NPs的胞吞作用。令人鼓舞的是,与未修饰的NP相比,25%R8 + 75%Cys NP在体内也表现出促进的肠道渗透和口服吸收。口服胰岛素25%R8 + 75%Cys NPs在糖尿病大鼠中的降血糖作用更强。总之,这项工作提供了一种符合高尔基体生理功能的分泌策略,以促进NP的胞吐作用,从而进一步提高了负载蛋白药物的口服吸收率。
更新日期:2021-01-26
中文翻译:

符合高尔基体对分泌性胞吐作用的生理功能,促进蛋白质药物的口服吸收
肠上皮细胞是口服纳米制剂和所输送蛋白药物的主要生物屏障。其中,除了细胞摄取外,细胞内运输途径和相关的胞吐作用对于载有药物的NP的跨上皮运输非常重要。在此,受高尔基体从细胞分泌蛋白质的生理功能启发,高尔基体定位相关氨基酸L-半胱氨酸(Cys)在NP的表面进行了修饰,以查看这种修饰是否以及如何指导高尔基途径相关的运输并促进载药NP的胞吐作用。同时,将穿透细胞的肽八精氨酸(R8)共修饰以增加细胞摄取。探索了R8和Cys修饰的比例,以获得NP的内吞作用和胞吐作用的最佳效果。结果,具有最多Cys修饰的25%R8 + 75%Cys NPs表现出高效的胞吞作用,具有最高的胞吞作用/胞吞作用比率(0.87)。有趣的是,胞吐机制研究表明,由于Cys修饰,它们通过高尔基体分泌途径贩运并绕过了溶酶体。详细的高尔基体定位机制研究进一步表明,来自Cys的巯基对介导高尔基体运输很重要。特别地,竞争性抑制研究表明,Cys修饰的NP在通过高尔基体分泌途径转运后,更有助于其胞吐作用。我们证明货物已经运输通过高尔基体的装置倾向于从细胞中运出并避免降解,这有助于体外25%R8 + 75%Cys NPs的胞吞作用。令人鼓舞的是,与未修饰的NP相比,25%R8 + 75%Cys NP在体内也表现出促进的肠道渗透和口服吸收。口服胰岛素25%R8 + 75%Cys NPs在糖尿病大鼠中的降血糖作用更强。总之,这项工作提供了一种符合高尔基体生理功能的分泌策略,以促进NP的胞吐作用,从而进一步提高了负载蛋白药物的口服吸收率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号