Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2021-01-26 , DOI: 10.1016/j.jece.2021.105129 Ching-Yao Hu , Wen-Hui Kuan , I-Ju Lee , Yu-Jung Liu
|
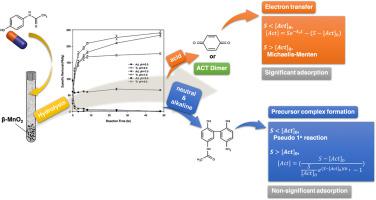
Acetaminophen is commonly found in aquatic environments because it is widely used as an analgesic. In this study, the extent of transformation and adsorption of acetaminophen by manganese dioxide (MnO2) at different pH conditions was systematically investigated. The adsorption of acetaminophen by MnO2 was significant under acidic conditions but negligible under neutral and alkaline conditions. This indicates that the rate-determining steps in the removal of acetaminophen are electron transfer under acidic conditions and precursor complex formation under neutral and alkaline conditions. The kinetic simulation result is consistent with the above results, and the kinetics can be described using a surface complexation and transformation kinetic model if the initial concentration of acetaminophen exceeds the total concentration of the reactive surface sites on MnO2. Otherwise, the Michaelis–Menten model and the pseudo-first-order kinetic model can be used to describe the removal kinetics under acidic conditions and under neutral and alkaline conditions, respectively. The main transformation products were p-benzoquinone and a dimer of 4-aminophenol and acetaminophen under acidic and alkaline conditions, respectively. This indicates that dehydration–oxidation is the main pathway only under acidic conditions. Under alkaline conditions, the main transformation mechanism is dimerization–hydrolysis.
中文翻译:

pH依赖性二氧化锰去除对乙酰氨基酚的机理和动力学
对乙酰氨基酚通常在水生环境中发现,因为它被广泛用作止痛药。在这项研究中,系统地研究了在不同pH条件下二氧化锰(MnO 2)对乙酰氨基酚的转化和吸附程度。MnO 2吸附对乙酰氨基酚在酸性条件下显着,但在中性和碱性条件下可忽略不计。这表明除去对乙酰氨基酚的决定速率的步骤是在酸性条件下的电子转移和在中性和碱性条件下前体复合物的形成。动力学模拟结果与上述结果一致,如果对乙酰氨基酚的初始浓度超过MnO 2上反应性表面位点的总浓度,则可以使用表面络合和转化动力学模型描述动力学。。否则,Michaelis–Menten模型和伪一级动力学模型可以分别用来描述酸性条件下以及中性和碱性条件下的去除动力学。在酸性和碱性条件下,主要的转化产物分别是对苯醌和4-氨基苯酚和对乙酰氨基酚的二聚体。这表明只有在酸性条件下,脱水氧化才是主要途径。在碱性条件下,主要的转化机理是二聚水解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号